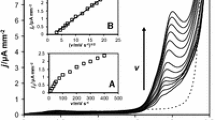
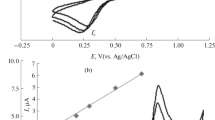
Abstract
Bare boron-doped diamond electrode (BDDE) was applied for investigation of deposition/strip** processes of silver ions in various supporting electrolytes. The highest signal was observed in 0.1 mol dm−3 HNO3. Detection of silver ions is also possible in 0.1 mol dm−3 Na2S2O3 where Ag+ is strongly complexed as [Ag(S2O3)2]3−. Though the sensitivity is 100 times lower, the potential of anodic dissolution of silver is significantly shifted towards the negative values. This shift might be useful for solving of some interferences which may occur in the detection process. After preliminary cyclic voltammetry, the analytical performance was studied by differential pulse anodic strip** voltammetry (DPASV) and square-wave anodic strip** voltammetry. Deposition potential of − 0.18 V vs. Ag/AgCl and deposition time of 240 s were selected as optimum DPASV parameters. The lowest detection limit of 2.0 × 10–10 mol dm−3 was achieved with DPASV in HNO3. Negligible effect of possible interferents on Ag response proved to be a good selectivity of method. The proposed method after validation was also applied to real samples analysis of some commercial products with complex matrix. The obtained results are statistically identical with data declared by manufacturer and gained by independent technique, making this method suitable for commercial product control.
Graphic abstract







Similar content being viewed by others
References
Guo W, Hu S, Zhang J, Zhang H (2011) Sci Total Environ 409:2981
Peng JJY, Botelho MG, Matinlinna JP (2012) J Dent 40:531
Fewtrell L (2014) Cent Res Environ Health 1
Pantic I (2014) Rev Adv Mater Sci 37:15
António DC, Cascio C, Jakšić Ž, Jurašin D, Lyons DM, Nogueira AJA, Rossi F, Calzolai L (2015) Mar Environ Res 111:162
Chaloupka K, Malam Y, Seifalian AM (2010) Trends Biotechnol 28:580
Mikelova R, Baloun J, Petrlova J, Adam V, Havel L, Petrek J, Horna A, Kizek R (2007) Bioelectrochemistry 70:508
Sang S, Yu C, Li N, Ji Y, Zhang J (2012) Int J Electrochem Sci 7:3306
Nadiki HH, Taher MA, Ashkenani H, Sheikhshoai I (2012) Analyst 137:2431
Araújo CST, Alves VN, Rezende HC, Coelho NMM (2010) Microchem J 96:82
Zhang T, Chai Y, Yuan R, Guo J (2012) Mater Sci Eng C 32:1179
Veverková L, Hradilová Š, Milde D, Panáček A, Skopalová J, Kvítek L, Petrželová K, Zbořil R (2014) Spectrochim Acta Part B 102:7
Hosoba M, Oshita K, Katarina RK, Takayanagi T, Oshima M, Motomizu S (2009) Anal Chim Acta 639:51
Anekthirakun P, Imyim A (2019) Microchem J 145:470
Choleva TG, Tsogas GZ, Giokas DL (2019) Talanta 196:255
Ashkenani H, Taher MA (2012) Microchem J 103:185
Zhang S, Pu Q, Liu P, Sun Q, Su Z (2002) Anal Chim Acta 452:223
Yang X, Jia Z, Yang X, Li G, Liao X (2017) Saudi J Biol Sci 24:589
López-López JA, Herce-Sesa B, Moreno C (2016) Talanta 159:117
Kocúrová L, Balogh IS, Nagy L, Billes F, Simon A, Andruch V (2011) Microchem J 99:514
Shah RK (2016) Orient J Chem 32:499
Ensafi AA, Zarei K (1997) Fresenius J Anal Chem 358:475
Ensafi AA, Abbasi AS (1997) Anal Lett 30:327
Jones P, Beere HG (1995) Anal Proc Incl Anal Commun 32:169
El-Mai H, Espada-Bellido E, Stitou M, García-Vargas M, Galindo-Riaño MD (2016) Talanta 151:14
Radulescu MC, Chira A, Radulescu M, Bucur B, Bucur MP, Radu GL (2010) Sensors 10:11340
Zejli H, de Cisneros JLHH, Naranjo-Rodriguez I, Temsamani KR (2007) Talanta 71:1594
Gholami M, Koivisto B (2019) Appl Surf Sci 467–468:112
Nezhadali A, Bonakdar GA (2019) J Food Drug Anal 27:305
Zhai X, Li Z, Shi J, Huang X, Sun Z, Zhang D, Zou X, Sun Y, Zhang J, Holmes M, Gong Y, Povey M, Wang S (2019) Food Chem 290:135
Min SH, Lee GY, Ahn SH (2019) Compos B 161:395
Dou N, Zhang S, Xu J (2019) RSC Adv 9:31440
Apyari VV, Terenteva EA, Kolomnikova AR, Garshev AV, Dmitrienko SG, Zolotov YA (2019) Talanta 202:51
Orouji A, Abbasi-Moayed S, Hormozi-Nezhad MR (2019) Spectrochim Acta Part A 219:496
Eksin E, Erdem A, Fafal T, Kivcak B (2019) Electroanalysis 31:1075
Culková E, Lukáčová-Chomisteková Z, Bellová R, Melicherčíková D, Durdiak J, Timko J, Rievaj M, Tomčík P (2018) Int J Electrochem Sci 13:6358
Hutton LA, Newton ME, Unwin PR, Macpherson JV (2011) Anal Chem 83:735
Banks CE, Hyde ME, Tomčík P, Jacobs R, Compton RG (2004) Talanta 62:279
Jahandari S, Taher MA, Fazelirad H, Sheikhshoai I (2013) Microchim Acta 180:347
Sadok I, Tyszczuk-Rotko K (2018) J Electroanal Chem 808:204
Ebrahimi M, Raoof JB, Ojani R (2015) Talanta 144:619
Li YH, **e HQ, Zhou FQ (2005) Talanta 67:28
Kamenev AI, Lushov KA (2001) J Anal Chem 56:380
Rohani T, Taher MA (2010) Talanta 80:1827
Acknowledgements
This study was supported by the GAPF Agency of Faculty of Education, The Catholic University in Ružomberok under the project No. GAPF 1/4/2019.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Culková, E., Lukáčová-Chomisteková, Z., Bellová, R. et al. Voltammetric detection of silver in commercial products on boron doped diamond electrode: strip** at lowered potential in the presence of thiosulfate ions. Monatsh Chem 151, 1009–1017 (2020). https://doi.org/10.1007/s00706-020-02634-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00706-020-02634-1