Abstract
Porcine reproductive and respiratory syndrome virus (PRRSV) is a pathogen that severely disrupts swine production. Despite sustained efforts, the disease is still endemic, with high mortality and morbidity. New antiviral strategies to control PRRSV are needed. Griffithsin, a red algal lectin, has potent antiviral effect on several human enveloped viruses, but this effect has not been demonstrated on PRRSV. Here, we first tested the in vitro antiviral activity of Griffithsin against PRRSV. Griffithsin exerted strong saccharide-dependent antiviral activity against PRRSV, probably through interactions with glycans on the surface of PRRSV that interfered with virus entry. Furthermore we revealed that Griffithsin’s action on PRRSV involved blocking viral adsorption, and it had no effect on viral penetration. Besides Our findings also suggested that Griffithsin may interfere with cell-to-cell spread to prevent virus transmission. The remarkable potency profile of Griffithsin supports its potential value as an antiviral agent against PRRSV.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Infection by porcine reproductive and respiratory syndrome virus (PRRSV) causes devastating disease in swine, resulting in significant economic losses estimated at $644 million per year in the United States [1]. PRRSV is an enveloped, single-stranded positive-sense RNA virus. Its 15.4 kb genome contains at least 10 open reading frames [2], four of which encode envelope glycoproteins designated GP2a, GP3, GP4 and GP5. GP5 has been proposed as the major mediator of PRRSV entry into target cells.
PRRSV infection can cause respiratory distress, reproductive dysfunction and weight loss. Moreover, PRRSV infection confers susceptibility to secondary infection by other viruses or bacteria. Vaccination is the most common prophylactic measure used to protect pigs against PRRSV infection. Currently, modified live virus (MLV) PRRSV vaccines are considered most effective although probably because of PRRSV genetic variation, MLV vaccines have failed to confer complete protection against drift variants [3, 4]. Thus, there is a need for novel antiviral drugs that could be used for prevention and control of PRRSV infection.
Griffithsin, derived from Griffithsia spp. marine red algae., is a small lectin consisting of 121 amino acids [5]. Griffithsin is a domain-swapped dimer and each subunit has three nearly equivalent glycan-binding sites [6]. Griffithsin binds glycan moieties associated with the glycoproteins of several enveloped viruses [7], resulting in inhibition of infectivity. Griffithsin has been shown to exhibit significant antiviral activity against human enveloped viruses including HIV [5, 8], Middle East respiratory syndrome coronavirus (MERS-CoV) [9], severe acute respiratory syndrome corona virus (SARS-CoV) [6, 10], hepatitis C virus (HCV) [11, 12], herpes simplex virus 2 (HSV-2) [13] and Japanese encephalitis virus (JEV) [14, 15]. Moreover, Griffithsin shows excellent thermostability [16], remaining stable up to 80°C, and is resistant to organic solvents [5] and protease degradation [17]. The cytotoxicity of Griffithsin has been studied extensively [18, 19], showing that it possesses a superior safety profile: no cytotoxicity was observed against a variety of cell types, nor any major effects on peripheral blood mononuclear cell activation or cytokine and chemokine production. Thus, Griffithsin is an attractive candidate for development as an antiviral therapeutic.
In this study, we assessed the antiviral activity of Griffithsin against PRRSV in Marc-145 cells. Our results revealed that Griffithsin could effectively reduce PRRSV infection by blocking virus adsorption, indicating that Griffithsin may be a promising antiviral agent for treatment of PRRSV.
Results
Secondary structure analysis using CD spectroscopy
The recombinant plasmid pUC57-Griffithsin was verified by restriction enzyme digestion (Fig. 1A) and recombinant Griffithsin was successfully expressed in E. coli and readily purified to homogeneity (Fig. 1B). To assess whether Griffithsin was well folded, we first studied its secondary structure using CD spectroscopy. The CD spectrum of Griffithsin displayed a maximum at 220.5 nm and a minimum at 199.5 nm (Fig. 2) which was consistent with a structure composed mainly of β-sheets. As shown in Table 1, Griffithsin secondary structure consisted mostly of β-sheets (51.1%) with roughly equal proportions of a-helices and turns.
Identification of recombinant plasmid pUC57-Griffithsin and SDS-PAGE analyses of Griffithsin. (A) Recombinant plasmid pUC57-Griffithsin was digested by NdeI–XhoI and verified in 1% Agarose Gel. (B) Recombinant Griffithsin was purified by Ni2+- Sepharose HisTrap HPTM affinity column by the analysis of Crude extract (Crude), Flow-through after loading crude extract on affinity column (FT), Fraction obtained by washing with 50 mM imidazole (Wash) and Fractions eluted with 300 mM imidazole.
Assessment of Griffithsin binding to OVA
The ability of Griffithsin to bind glycans was assessed by ELISA against immobilized OVA. OVA contains a single N-linked glycosylation site, in which bind to high-mannose and hybrid N-linked glycans have been characterized. ELISA measurements revealed that Griffithsin interacted with OVA in a dose-dependent manner. OVA binding by Griffithsin was efficiently inhibited by 100 mM mannose and slightly less efficiently inhibited by 100 mM glucose, indicating that Griffithsin bound mannose with higher affinity compared with glucose (Fig. 3).
Griffithsin inhibits PRRSV infection in vitro
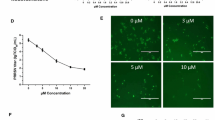
Immunofluorescence microscopy revealed that Griffithsin potently inhibited PRRSV infection of Marc-145 cells. As shown in Fig. 4.A, Griffithsin significantly decreased PRRSV infectivity in a dose-dependent manner, and this inhibition was substantially diminished in the presence of 100 mM mannose. Furthermore, the infection rate of 95.1% in the presence of 0 µg/mL Griffithsin, was reduced to 25.3% in the presence of 4 µg/mL Griffithsin when Marc-145 cells were infected with PRRSV at a MOI of 10 (Fig. 4.B). More significant reduction was observed when the cells were infected with 5 MOI PRRSV, where infection rate was reduced to 4.4% from 81.3% in the presence of 4 µg/mL Griffithsin (Fig. 4.B).
Griffithsin inhibits PRRSV infection of Marc-145 cells. (A) Indirect immunofluorescence assay of PRRSV-infected Marc-145 cells in the presence of Griffithsin. (B) Quantification of PRRSV infected cells with the treatment of Griffithsin. The results are expressed as percent-infected cells calculated from the number of infected cells (FITC stain) / total number of cells (DAPI nuclei stain) × 100 %. Each value represents the mean of three independent experiments and its standard derivation. *P < 0.05, **P < 0.01 and ***P < 0.001 compared to the 0 µg/mL control group.
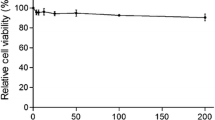
To understand whether these experimental results might instead be explained by a cytotoxic effect of Griffithsin, we assessed whether Griffithsin affected the proliferative activity of Marc-145 cells. As shown in Fig. 5, cells cultured in medium containing Griffithsin at the same concentrations as used in this study retained 100% viability compared with control cells.
Griffithsin prevents the adsorption stage of PRRSV infection
To elucidate the mechanism of the antiviral effects of Griffithsin, a virus entry assay was performed. The data indicated that Griffithsin greatly repressed virus adsorption to cells, with no effects on virus penetration.
Marc-145 cells were incubated with PRRSV and Griffithsin at 4°C in the presence or absence of mannose to allow virus adsorption but not penetration. As shown in Fig. 6, 4 µg/mL Griffithsin greatly repressed PRRSV adsorption but had no effect on virions treated with Griffithsin in the presence of mannose (P = 0.03). No significant impact on PRRSV infectivity was observed when Griffithsin was added during viral penetration (P > 0.05).
Effects of Griffithsin on viral load measured using RT-qPCR and western blot
RT-qPCR was performed to investigate the effect of Griffithsin on intracellular viral load. Marc-145 cells were incubated with PRRSV at a MOI of 5 for 24 h, and then growth media was then replaced with fresh medium containing 4 µg/mL Griffithsin. Abundance of PRRSV GP5 RNA in Marc-145 cells was analyzed at different times post-Griffithsin treatment. As shown in Fig. 7.A, compared with the corresponding untreated control group, PRRSV RNA levels in the Griffithsin-treated group showed the highest decreases at 12 h (P < 0.001), 24 h (P < 0.001), and 36 h (P < 0.001). However, no significant differences in RNA abundance were observed at 6 h post-Griffithsin addition (P > 0.05). We also measured levels of PRRSV GP5 protein in Marc-145 cells using western blot after 36 h Griffithsin treatment. The results showed that expression of PRRSV GP5 was significantly reduced following Griffithsin treatment in a dose-dependent manner (Fig. 7.B).
Influence of Griffithsin on total PRRSV RNA and protein levels in Marc-45 cells. (A) Level of PRRSV RNA after treatment was determined by RT-qPCR at different time points post-infection with Griffithsin-treated virions. Relative expression (fold change) in comparison with a control group not treated with Griffithsin (denoted as 1) is illustrated. Data are presented as means ± standard deviations of three independent experiments. *P < 0.05; **P < 0.01; ***P < 0.001 compared to the control group. (B) The PRRSV GP5 protein and β-actin were detected by western blot. β-actin was used as the internal control.
Agglutination of Griffithsin
Since Griffithsin belongs to a group of lectins that potentially induce hemagglutination, so we determined whether Griffithsin had any hemagglutination activity. We tested blood cells derived from several species including avian, swine, goat, guinea pig and mice, and the results are shown in Fig. 8. The red cells of most species ’ red blood cells were not agglutinated by Griffithsin. with the exception of guinea pig red blood cells, which were agglutinated in the presence of Griffithsin when used at a concentrations higher than 12.5 µg/mL.
Discussion
Griffithsin is currently considered the most potent anti-HIV agent for blocking virus entry by interacting with with the Env glycoprotein on the surface of HIV, exhibiting antiviral activity at concentrations in the nanomolar to picomolar range [8]. However, the antiviral activity of Griffithsin against PRRSV has not been investigated . PRRSV, like HIV, is an enveloped virus. and its major envelope glycoprotein, GP5, is heavily glycosylated [23, 24]. We analyzed therefore the potential ability of Griffithsin to inhibit infection by PRRSV, with the hope of develo** a novel antiviral strategy for PRRSV therapy.
To further study its antiviral activity against enveloped viruses of veterinary importance, biologically active recombinant Griffithsin was produced in E. coli with high expression yields. Our data indicated that the major secondary structure elements of Griffithsin were β-sheets, consistent with previous reports [6, 25], indicating that its overall fold was probably similar to that of natural Griffithsin. Additional ELISA experiments demonstrated that the glycan-binding ability of Griffithsin, which was critical for its antiviral activity and further evaluated the anti-PRRSV activity of Griffithsin.
Our results showed that Griffithsin possessed potent antiviral activity against PRRSV in Marc-145 cells. Previous studies have been reported that certain lectins like porcine SP-A are able to inhibit inhibited PRRSV infection [26,27,28,29,30]. . Interestingly, Griffithsin was found to reduce PRRSV infectivity of Marc-145 cells at a 7.5-fold lower concentrations and at a 500-fold higher MOI compared with porcine SP-A [20]. Porcine ficolin (10 µg/mL) which is also a lectin a lectin has also been shown to inhibit PRRSV infection [31]. However, our previous work demonstrated that solubility and low expression represented were the main obstacles to large-scale cost-effective production of biologically active ficolin. Finally, Although many other antivirals against PRRSV have been described in the literature, none have been able to effectively treat or prevent PRRSV infection in vivo for whatever reason .and Griffithsin may thus represent a candidate agent for preventing or treating PRRSV infection.
Another key finding of our study was that Griffithsin inhibited infection by PRRSV using a higher dose than for HIV strains, but at lower than that required those for SARS-CoV strains [7]. Since the HIV Env glycoprotein is heavily glycosylated containing up to 50% carbohydrate by weight [8, 32], this may explain Griffithsin’s potent anti-HV activity. The antiviral properties of Griffithsin against enveloped viruses is thus likely to be related to the glycosylation of viral proteins, a hypothesis that should be further investigated by further studies.
Most interestingly, our results clearly showed that Griffithsin was able to block virus adsorption but had only modest effects on virus penetration, which is consistent with most previous reports. Millet and colleagues performed detailed studies to define the viral life cycle stage at which Griffithsin acted and found that Griffithsin inhibited MERS-CoV by acting on the adsorption stage [9]. By contrast, Griffithsin exhibited its antiviral activity against HSV-2 by preventing cell-to-cell spread, but had little effect on HSV-2 entry into target cells [33]. The reason for this difference may be related to the virus itself and its entry receptors. Further studies are needed to clarify the anti-PRRSV activity of Griffithsin in porcine alveolar macrophages, the main targets of PRRSV infection in vivo.
Our data clearly showed that Griffithsin displayed its antiviral activity in a saccharide-dependent manner. Taking our own findings and the results of previous studies together, we inferred that the binding of Griffithsin to PRRSV glycoproteins prevented PRRSV attachment to target cells, possibly due to steric hindrance or loss of PRRSV receptor-binding ability. Previous studies revealed that Griffithsin reduced HIV, HCV, SARS-CoV, MERS-CoV, and JEV infectivity through binding to viral glycoproteins, thereby blocking viral entry. It was also confirmed that Griffithsin could bind to glycoprotein of HSV-2 and inhibited viral transmission by blocking cell-to-cell spread. Interestingly, Griffithsin also has antiviral activity against HPV, a non-enveloped virus, by binding to the secondary receptor α6 integrin and decreasing its availability on the cell surface [7]. Due to the uncertain nature of the interactions between Griffithsin, PRRSV and host cells, its mode of action should be clarified in future studies.
Our study also showed that Griffithsin greatly reduced total viral RNA 12 h, 24 h and 36 h post-infection, but had no effect at 6 h post-infection, after the initial rounds of viral replication. This finding suggested that Griffithsin may neutralize progeny virions and/or interfere with cell-to-cell spread, a possibility which needs to be studied by future work.
In conclusion, our study provides the first evidence of the antiviral activity of Griffithsin against PRRSV. Our results revealed that Griffithsin exerted a potent inhibitory effect on highly pathogenic PRRSV by interfering with viral entry and/or cell-to-cell spread. Thus, Griffithsin may seems to be a candidate agent for preventing PRRSV infection and further studies in live animals are necessary to confirm its value for inhibiting PRRSV infection in vivo.
References
Kristensen C, Kvisgaard L, Pawlowski M, Holmgaard Carlsen S, Hjulsager C, Heegaard P, Bøtner A, Stadejek T, Haugegaard S, Larsen L (2017) Efficacy and safety of simultaneous vaccination with two modified live virus vaccines against porcine reproductive and respiratory syndrome virus types 1 and 2 in pigs. Vaccine 36(2):227–236
Chand RJ, Trible BR, Rowland RR (2012) Pathogenesis of porcine reproductive and respiratory syndrome virus. Curr Opin Virol 2:256–263
Renukaradhya GJ, Meng XJ, Calvert JG, Roof M, Lager KM (2015) Inactivated and subunit vaccines against porcine reproductive and respiratory syndrome: current status and future direction. Vaccine 33:3065–3072
Renukaradhya GJ, Meng XJ, Calvert JG, Roof M, Lager KM (2015) Live porcine reproductive and respiratory syndrome virus vaccines: current status and future direction. Vaccine 33:3065–3072
Mori T, O’Keefe BR, Bringans S, Gardella R, Berg S, Cochran P, Turpin JA Jr, Jr Mcmahon JBJR (2005) Isolation and characterization of Griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem 280:9345–9353
Ziolkowska N, O’Keefe B, Mori T, Zhu C, Giomarelli B, Vojdani F, Palmer K, Mcmahon JA (2006) Domain-swapped structure of the potent antiviral protein Griffithsin and its mode of carbohydrate binding. Structure 14:1127
Lusvarghi S, Bewley CA (2016) Griffithsin: an antiviral lectin with outstanding therapeutic potential. Viruses 8:296
Xue J (2014) Investigation of the mechanism of Griffithsin (GRFT): a potent HIV entry inhibitor. Dissertations & Theses-Gradworks
Millet JK, Séron K, Labitt RN, Danneels A, Palmer KE, Whittaker GR, Dubuisson J, Belouzard S (2016) Middle East respiratory syndrome coronavirus infection is inhibited by Griffithsin. Antivir Res 133:1–8
O’Keefe BR, Giomarelli B, Barnard DL, Shenoy SR, Chan PKS, Mcmahon JB, Palmer KE, Barnett BW, Meyerholz DK, Wohlfordlenane CL (2010) Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein Griffithsin against emerging viruses of the family Coronaviridae. J Virol 84:2511–2521
Meuleman P, Albecka A, Belouzard S, Vercauteren K, Verhoye L, Wychowski C, Lerouxroels G, Palmer KE, Dubuisson J (2011) Griffithsin has antiviral activity against hepatitis C virus. Antimicrob Agents Chemother 55:5159–5167
Takebe Y, Saucedo CJ, Lund G, Uenishi R, Hase S, Tsuchiura T, Kneteman N, Ramessar K, Tyrrell DL, Shirakura M (2013) Antiviral lectins from red and blue-green algae show potent in vitro and in vivo activity against hepatitis C virus. PLoS One 8:e64449
Levendosky K, Mizenina O, Martinelli E, Jeanpierre N, Kizima L, Rodriguez A, Kleinbeck K, Bonnaire T, Robbiani M, Zydowsky TM (2015) Griffithsin and carrageenan combination to target herpes simplex virus 2 and human papillomavirus. Antimicrob Agents Chemother 59:7290–7298
Ishag HZ, Li C, Wang F, Mao X (2016) Griffithsin binds to the glycosylated proteins (E and prM) of Japanese encephalitis virus and inhibit its infection. Virus Res 215:50–54
Ishag HZA, Li C, Huang L, Sun MX, Wang F, Ni B, Malik T, Chen PY, Mao X (2013) Griffithsin inhibits Japanese encephalitis virus infection in vitro and in vivo. Arch Virol 158:349–358
Fuqua JL, Wanga V, Palmer KE (2015) Improving the large scale purification of the HIV microbicide, Griffithsin. BMC Biotechnol 15:12
Moncla BJ, Pryke K, Rohan LC, Graebing PW (2011) Degradation of naturally occurring and engineered antimicrobial peptides by proteases. Adv Biosci Biotechnol 2:404–408
Kouokam JC, Lasnik AB, Palmer KE (2016) Studies in a murine model confirm the safety of Griffithsin and advocate its further development as a microbicide targeting HIV-1 and other enveloped viruses. Viruses 8:311
Kouokam JC, Huskens D, Schols D, Johannemann A, Riedell SK, Walter W, Walker JM, Matoba N, O’Keefe BR, Palmer KE (2011) Investigation of Griffithsin’s interactions with human cells confirms its outstanding safety and efficacy profile as a microbicide candidate. PLoS One 6:e22635
Li L, Zheng Q, Zhang Y, Li P, Fu Y, Hou J, **ao X (2016) Antiviral activity of recombinant porcine surfactant protein A against porcine reproductive and respiratory syndrome virus in vitro. Arch Virol 161:1883–1890
Urtasun N, Baieli MF, Cascone O, Wolman FJ, Miranda MV (2015) High-level expression and purification of recombinant wheat germ agglutinin in Rachiplusia nu larvae. Process Biochem 50:40–47
Woodrum BW, Maxwell J, Allen DM, Wilson J, Krumpe LR, Bobkov AA, Hill RB, Kibler KV, O’Keefe BR, Ghirlanda G (2016) A designed, “Nested” dimer of cyanovirin-N increases antiviral activity. Viruses 8:158
Li J, Tao S, Orlando R, Murtaugh MP (2015) N-glycosylation profiling of porcine reproductive and respiratory syndrome virus envelope glycoprotein 5. Virology 478:86–98
Li J, Murtaugh MP (2015) Functional analysis of porcine reproductive and respiratory syndrome virus N-glycans in infection of permissive cells. Virology 477:82–88
Micewicz ED, Cole AL, Jung CL, Luong H, Phillips ML, Pratikhya P, Sharma S, Waring AJ, Cole AM, Ruchala P (2010) Grifonin-1: a small HIV-1 entry inhibitor derived from the algal lectin. Griffithsin. Plos One 5:e14360
Duan E, Wang D, Fang L, Ma J, Luo J, Chen H, Li K, **ao S (2015) Suppression of porcine reproductive and respiratory syndrome virus proliferation by glycyrrhizin. Antivir Res 120:122–125
Li E, Sun N, Zhao JX, Sun YG, Huang JG, Lei HM, Guo JH, Hu YL, Wang WK, Li HQ (2015) In vitro evaluation of antiviral activity of tea seed saponins against porcine reproductive and respiratory syndrome virus. Antivir Ther 20:743
Feng J, Bai X, Cui T, Han Z, Yao C, **e J, Shi Q, Wang H, Zhang G (2016) In vitro antiviral activity of germacrone against porcine reproductive and respiratory syndrome virus. Curr Microbiol 73:1–7
Evans AB, Dong P, Loyd H, Zhang J, Kraus GA, Carpenter S (2017) Identification and characterization of small molecule inhibitors of porcine reproductive and respiratory syndrome virus. Antivir Res 146:28–35
Yang Q, Gao L, Si J, Sun Y, Liu J, Cao L, W-h Feng (2013) Inhibition of porcine reproductive and respiratory syndrome virus replication by flavaspidic acid AB. Antivir Res 97:66–73
Keirstead ND, Lee C, Yoo D, Brooks AS, Hayes MA (2008) Porcine plasma ficolin binds and reduces infectivity of porcine reproductive and respiratory syndrome virus (PRRSV) in vitro. Antivir Res 77:28–38
Balzarini J (2006) Large-molecular-weight carbohydrate-binding agents as HIV entry inhibitors targeting glycoprotein gp120. Curr Opin HIV AIDS 1:355–360
Nixon B, Stefanidou M, Mesquita PM, Fakioglu E, Segarra T, Rohan L, Halford W, Palmer KE, Herold BC (2013) Griffithsin protects mice from genital herpes by preventing cell-to-cell spread. J Virol 87:6257–6269
Acknowledgements
This study was supported by grants from the Special Fund for Agro-scientific Research in the Public Interest (No. 201303046). The erythrocytes were kindly provided by Yiwei Wang.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Additional information
Handling Editor: Zhenhai Chen.
Rights and permissions
About this article
Cite this article
Li, L., Tian, X., Chen, J. et al. Griffithsin inhibits porcine reproductive and respiratory syndrome virus infection in vitro. Arch Virol 163, 3317–3325 (2018). https://doi.org/10.1007/s00705-018-4029-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-018-4029-x