Abstract
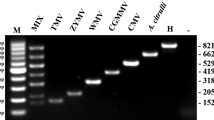
Pathogens causing significant economic losses in chrysanthemum include tomato aspermy virus (TAV), chrysanthemum virus B (CVB), cucumber mosaic virus (CMV), tobacco mosaic virus (TMV), potato virus Y (PVY), chrysanthemum stunt viroid (CSVd) and chrysanthemum chlorotic mottle viroid (CChMVd). A multiplex reverse transcription polymerase chain reaction (RT-PCR) method, using specific primer sets for each virus or viroid, was developed for simultaneous detection and differentiation of TAV, CVB, CMV, TMV, PVY, CChMVd, and CSVd. The RT-PCR method was validated by testing chrysanthemum samples collected from different regions of China. In this study, CVB, TAV, TMV, PVY, CSVd, CMV, and CChMVd were detected, respectively, in 24.7 %, 17.5 %, 4.4 %, 4.4 %, 2.9 %, 2.5 %, and 1.5 % of the samples tested. These results indicate that CVB and TAV (24.7 % and 17.5 %) are common, whereas CMV, TMV, CChMVd, CSVd, and PVY (all below 5 %) are less frequently encountered. This new multiplex RT-PCR method has potential to be used routinely in large-scale virus and viroid surveys.



Similar content being viewed by others
References
Verhoeven JTJ, Roenhorst JW, Cortes I, Peters D (1996) Detection of a novel tospovirus in chrysanthemum. Acta Hort 432:44–51
Chen TM, Chen YK, Chen MJ, Ye SD (1999) Host reactions, cytological characteristics and serological properties of a Turnip mosaic virus isolate causing albinic mosaic disease of garland chrysanthemum. Plant Pathol Bull 8:73–82
Megan FH, Giles RJ, Moran JR, Hepworth G (2001) The incidence of Chrysanthemum stunt viroid, Chrysanthemum B carlavirus, Tomato aspermy cucumovirus and Tomato spotted wilt tospovirus in Australian chrysanthemum crops. Plant Pathol 25:174–178
Farzadfar Sh, Ohshima K, Pourrahim R, Golnaraghi AR, Jalali S, Ahoonmanesh A (2005) Occurrence of Turnip mosaic virus on ornamental crops in Iran. Plant Pathol 54:261
Toru K, Kazuo Y, Satoru S (2011) First report of impatiens necrotic spot virus infecting chrysanthemum (Chrysanthemum morifolium) in Japan. J Gen Plant Patho 77:263–265
Song A, You Y, Chen F, Li P, Jiang J, Chen S (2012) A multiplex RT-PCR for rapid and simultaneous detection of viruses and viroids in chrysanthemum. Lett Appl Microbiol 56:8–13
Raj SK, Kumar S, Choudhari S (2007) Identification of Tomato Aspermy Virus as the cause of yellow mosaic and flower deformation of chrysanthemums in India. Australas Plant Dis Notes 2:1–2
Noordam D (1952) Virus ziektenbij chrysant in Nedeland. With a summary: virus disease of Chrysanthemum morifolium in Netherlands. Tijdschrift over Plantenzikten 58:121–190
Singh L, Hallan V, Jabeen N, Singh AK, Ram R, Martin DP, Zaidi AA (2007) Coat protein gene diversity among Chrysanthemum virus B isolates from India. Arch Virol 152:405–413
Kumar S, Khan MS, Raj SK, Sharma AK (2009) Elimination of mixed infection of Cucumber mosaic and Tomato aspermy virus from chrysanthemum morifolium Ramat. cv. Pooja by shoot meristem culture. Sci Hortic 119:108–112
King AMQ, Lefkowitz E, Adams MJ, Carstens EB (2011) Virus taxonomy: ninth report of the international committee on taxonomy of viruses. Elsevier, The Netherlands
Navarro B, Gisel A, Rodio ME, Delgado S, Flores R, di Serio F (2012) Viroids: how to infect a host and cause disease without encoding proteins. Biochimie 94:1474–1480
Cho WK, Jo Y, Jo KM, Kim KH (2013) A current overview of two viroids that infect chrysanthemums: Chrysanthemum stunt viroid and Chrysanthemum chlorotic mottle viroid. Viruses 5:1099–1113
Palukaitis P, Symons RH (1980) Purification and characterization of the circular and linear forms of Chrysanthemum stunt viroid. J Gen Virol 46:477–489
Dimock AW, Geissinger CM (1969) A newly recognized disease of chrysanthemum caused by a graft-transmissible agent. Phytopathology 59:1024
Yamamoto H, Sano T (2005) Occurrence of Chrysanthemum chlorotic mottle viroid in Japan. J Gen Plant Pathol 71:156–157
Zhang ZX, Pan S, Li SF (2011) First Report of Chrysanthemum chlorotic mottle viroid in chrysanthemum in China. Plant Dis 95:1320
Haseloff J, Symons RH (1981) Chrysanthemum stunt viroid: Primary sequence and secondary structure. Nucleic Acids Res 9:2741–2752
Bostan H, Gazel M, Elibuyuk IO, Calayan K (2012) Occurrence of Pospiviroid in potato, tomato and some ornamental plants in Turkey. Afr J Biotechnol 9:2613–2617
Matsushitali Y (2007) Nucleotide sequences and distribution of Chrysanthemum stunt viroid in Japan. J Japan Soc Hort Sci 76:333–337
Chung BN, Lim JH, Choi SY, Kim JS, Lee EJ (2005) Occurrence of Chrysanthemum stunt viroid in chrysanthemum in Korea. Plant Pathol J 21:377–382
Singh D, Pathania M, Ram R, Zaidi AA, Verma N (2010) Screening of chrysanthemum cultivars for Chrysanthemum stunt viroid in an Indian scenario. Arch Phytopathol Plant Protect 43:1517–1523
Hiruki C (1970) Red kidney bean, a useful bioassay host for qualitative and quantitative work with potato virus M. Phytopathology 60:739–740
Verma N, Kumar K, Kulshrestha S, Raikhy G, Hallan V, Ram R, Zaidi AA, Garg ID (2009) Molecular studies on Tomato aspermy virus isolates infecting chrysanthemum. Arch Phytopathol Plant Protect 42:99–111
Fulladolsa Palma AC, Kota R, Charkowski AO (2013) Optimization of a chemiluminescent dot-blot immunoassay for detection of potato viruses. Am J Potato Res 90:306–312
Letschert B, Adam G, Lesemann D, Willingmann P, Heinze C (2002) Detection and differentiation of serologically cross-reacting tobamoviruses of economical importance by RT-PCR and RT-PCR-RFLP. J Virol Methods 106:1–10
Sharman M, Thomas JE, Dietzgen RG (2000) Development of a multiplex immunocapture PCR with colourimetric detection for viruses of banana. J Virol Methods 89:75–88
Blanco-Urgoiti B, Sánchez F, Dopazo J, Ponz F (1996) A strain-type clustering of potato virus Y based on the genetic distance between isolates calculated by RFLP analysis of the amplified coat protein gene. Arch Virol 141:2425–2442
Park J, Jung Y, Kil EJ, Kim J, Tran DT, Choi SK, Yoon JY, Cho WK, Lee S (2013) Loop-mediated isothermal amplification for the rapid detection of Chrysanthemum chlorotic mottle viroid (CChMVd). J Virol Methods 193:232–237
Ragozzino E, Faggioli F, Barba M (2004) Development of a one tube-one step RT-PCR protocol for the detection of seven viroids in four genera: Apscaviroid, Hostuviroid, Pelamoviroid and Pospiviroid. J Virol Methods 121:25–29
Uga H, Tsuda S (2005) A one-step reverse transcription polymerase chain reaction system for the simultaneous detection and identification of multiple Tospovirus infection. Phytopathology 95:166–171
Ge BB, Li Q, Liu GJ, Lu MG, Li SF, Wang HQ (2013) Simultaneous detection and identification of four viruses infecting pepino by multiplex RT-PCR. Arch Virol 158:1181–1187
Yan CN, Wang YQ, Yang Q, Zhang XH, Wu ZY, Huang CL (2009) Identification of Chrysanthemum virus B and Tomato aspermy virus by double RT-PCR. Plant Protect 35:89–90
Rybicki EP, Pietersen G (1999) Plant virus disease problems in the develo** world. Adv Virus Res 53:127–175
Marani F (1969) Chrysanthemum aspermy virus(CAV). Phytopath Med 8:142–149
Chuyan A, Krylov AV (1979) Properties of Tomato aspermy virus from chrysanthemum and its host range in the Primor’e region. Byu Glav Bot Sada 114:84–92
Gupta RP, Singh BP (1981) Studies on Chrysanthemum aspermy virus on chrysanthemum. Indian J Mycol Pl Pathol 11:158–160
Tao Y, Man JY, Wu YF (2012) Development of a multiplex polymerase chain reaction for simultaneous detection of wheat viruses and a phytoplasma in China. Arch Virol 157:1261–1267
Elnifro EM, Ashshi AM, Cooper RJ, Klapper PE (2000) Multiplex PCR: optimization and application in diagnostic virology. Clin Microbiol Rev 13:559–570
Acknowledgments
This work was supported by grants from the National Natural Science Foundation of China (No. 31171990 and No. 31372105).
Author information
Authors and Affiliations
Corresponding author
Additional information
X. Zhao and X. Liu contributed equally to this work.
Rights and permissions
About this article
Cite this article
Zhao, X., Liu, X., Ge, B. et al. A multiplex RT-PCR for simultaneous detection and identification of five viruses and two viroids infecting chrysanthemum. Arch Virol 160, 1145–1152 (2015). https://doi.org/10.1007/s00705-015-2360-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00705-015-2360-z