Abstract
Background
The c-Jun N-terminal kinase (JNK) proteins are encoded by three genes (JNK1, JNK2, and JNK3), giving rise to multiple isoforms via alternative splicing. JNK inhibition using a chemical inhibitor SP600125 confers neuroprotection in an animal model of subarachnoid hemorrhage (SAH). The aim of this study is to investigate whether the protective effects of SP600125 were associated with modulation of tight junction proteins including claudin-5 and ZO-1 and to define which JNK isoforms were involved in the early brain injury after SAH.
Methods
Seventy-five male Sprague Dawley rats (weighing 300–350 g) were randomly assigned to five groups (n = 15): (1) sham, (2) SAH, (3) SAH + DMSO (dimethyl sulfoxide), (4) SAH + 10 mg/kg SP600125, and (5) SAH + 30 mg/kg SP600125. SP600125 or DMSO was injected intraperitoneally 1 h before and 6 h after the induction of SAH. Animals from all the groups were killed 24 h after SAH, and brain tissues were dissected and subjected to electron microscopic examination, Western blot analysis, and histological evaluation.
Results
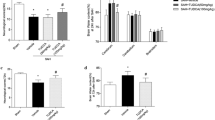
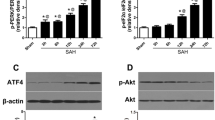
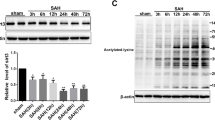
SP600125 pretreatment restored tight junctions and attenuated blood–brain barrier (BBB) disruption and cerebral edema after SAH, coupled with reduced apoptosis in the cerebral cortex. SP600125 exposure restored the reduced expression of both claudin-5 and ZO-1 following SAH and normalized the levels of JNK1 and JNK3.
Conclusion
Our data demonstrate that the JNK signaling plays an important role in the regulation of tight junction proteins and BBB integrity, and thus represents a promising target against brain injuries after SAH.





Similar content being viewed by others
References
Abbott NJ, Patabendige AA, Dolman DE, Yusof SR, Begley DJ (2010) Structure and function of the blood–brain barrier. Neurobiol Dis 37:13–25
Aveleira CA, Lin CM, Abcouwer SF, Ambrósio AF, Antonetti DA (2010) TNF-α signals through PKCζ/NF-κB to alter the tight junction complex and increase retinal endothelial cell permeability. Diabetes 59:2872–2882
Barnat M, Enslen H, Propst F, Davis RJ, Soares S, Nothias F (2010) Distinct roles of c-Jun N-terminal kinase isoforms in neurite initiation and elongation during axonal regeneration. J Neurosci 30:7804–7816
Bazzoni G (2006) Endothelial tight junctions: permeable barriers of the vessel wall. Thromb Haemost 95:36–42
Bederson JB, Germano IM, Guarino L (1995) Cortical blood flow and cerebral perfusion pressure in a new noncraniotomy model of subarachnoid hemorrhage in the rat. Stroke 26:1086–1091
Broderick JP, Brott TG, Duldner JE, Tomsick T, Leach A (1994) Initial and recurrent bleeding are the major causes of death following subarachnoid hemorrhage. Stroke 25:1342–1347
Brown RC, Davis TP (2002) Calcium modulation of adherens and tight junction function: a potential mechanism for blood–brain barrier disruption after stroke. Stroke 33:1706–1711
Bogoyevitch MA (2006) The isoform-specific functions of the c-Jun N-terminal Kinases (JNKs): differences revealed by gene targeting. Bioessays 28:923–934
Cahill J, Calvert JW, Zhang JH (2006) Mechanisms of early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 26:1341–1353
Crawford ED, Wells JA (2011) Caspase substrates and cellular remodeling. Annu Rev Biochem 80:1055–1087
Dhanasekaran DN, Reddy EP (2008) JNK signaling in apoptosis. Oncogene 27:6245–6251
Duris K, Manaenko A, Suzuki H, Rolland WB, Krafft PR, Zhang JH (2011) α7 Nicotinic acetylcholine receptor agonist PNU-282987 attenuates early brain injury in a perforation model of subarachnoid hemorrhage in rats. Stroke 42:3530–3536
Fanning AS, Jameson BJ, Jesaitis LA, Anderson JM (1998) The tight junction protein ZO-1 establishes a link between the transmembrane protein occludin and the actin cytoskeleton. J Biol Chem 273:29745–29753
Fontijn RD, Volger OL, Fledderus JO, Reijerkerk A, de Vries HE, Horrevoets AJ (2008) SOX-18 controls endothelial-specific claudin-5 gene expression and barrier function. Am J Physiol Heart Circ Physiol 294:H891–H900
Gupta S, Barrett T, Whitmarsh AJ, Cavanagh J, Sluss HK, Dérijard B, Davis RJ (1996) Selective interaction of JNK protein kinase isoforms with transcription factors. EMBO J 15:2760–2770
Hishikawa T, Ono S, Ogawa T, Tokunaga K, Sugiu K, Date I (2008) Effects of deferoxamine-activated hypoxia-inducible factor-1 on the brainstem after subarachnoid hemorrhage in rats. Neurosurgery 62:232–240
Kuan CY, Yang DD, Samanta Roy DR, Davis RJ, Rakic P, Flavell RA (1999) The Jnk1 and Jnk2 protein kinases are required for regional specific apoptosis during early brain development. Neuron 22:667–676
Kusaka G, Ishikawa M, Nanda A, Granger DN, Zhang JH (2004) Signaling pathways for early brain injury after subarachnoid hemorrhage. J Cereb Blood Flow Metab 24:916–925
László FA, Varga C, Dóczi T (1995) Cerebral oedema after subarachnoid haemorrhage. Pathogenetic significance of vasopressin. Acta Neurochir (Wien) 133:122–133
Liu J, Lin A (2005) Role of JNK activation in apoptosis: a double-edged sword. Cell Res 15:36–42
Li Y, Tang J, Khatibi NH, Zhu M, Chen D, Zheng W, Wang S (2010) Ginsenoside Rbeta1 reduces neurologic damage, is anti-apoptotic, and down-regulates p53 and BAX in subarachnoid hemorrhage. Curr Neurovasc Res 7:85–94
Matz PG, Fujimura M, Lewen A, Morita-Fujimura Y, Chan PH (2001) Increased cytochrome c-mediated DNA fragmentation and cell death in manganesesuperoxide dismutase-deficient mice after exposure to subarachnoid hemolysate. Stroke 32:506–515
Mikawa S, Kinouchi H, Kamii H, Gobbel GT, Chen SF, Carlson E, Epstein CJ, Chan PH (1996) Attenuation of acute and chronic damage following traumatic brain injury in copper, zinc-superoxide dismutase transgenic mice. J Neurosurg 85:885–891
Morita K, Sasaki H, Furuse M, Tsukita S (1999) Endothelial claudin: claudin-5/TMVCF constitutes tight junction strands in endothelial cells. J Cell Biol 147:185–194
Nag S, Kapadia A, Stewart DJ (2011) Review: molecular pathogenesis of blood–brain barrier breakdown in acute brain injury. Neuropathol Appl Neurobiol 37:3–23
Nitta T, Hata M, Gotoh S, Seo Y, Sasaki H, Hashimoto N, Furuse M, Tsukita S (2003) Size-selective loosening of the blood–brain barrier in claudin-5-deficient mice. J Cell Biol 161:653–660
Ostrowski RP, Colohan AR, Zhang JH (2005) Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab 25:554–571
Ostrowski RP, Colohan AR, Zhang JH (2006) Molecular mechanisms of early brain injury after subarachnoid hemorrhage. Neurol Res 28:399–414
Park JE, Lee JA, Park SG, Lee DH, Kim SJ, Kim HJ, Uchida C, Uchida T, Park BC, Cho S (2012) A critical step for JNK activation: isomerization by the prolyl isomerase Pin1. Cell Death Differ 19:153–161
Porter AG, Jänicke RU (1999) Emerging roles of caspase-3 in apoptosis. Cell Death Differ 6:99–104
Schievink WI, Riedinger M, Jhutty TK, Simon P (2004) Racial disparities in subarachnoid hemorrhage mortality: Los Angeles County, California, 1985–1998. Neuroepidemiology 23:299–305
Singh R, Wang Y, **ang Y, Tanaka KE, Gaarde WA, Czaja MJ (2009) Differential effects of JNK1 and JNK2 inhibition on murine steatohepatitis and insulin resistance. Hepatology 49:87–96
Suzuki H, Hasegawa Y, Kanamaru K, Zhang JH (2011) Mitogen-activated protein kinases in cerebral vasospasm after subarachnoid hemorrhage: a review. Acta Neurochir Suppl 110:133–139
Yan J, Li L, Khatibi NH, Yang L, Wang K, Zhang W, Martin RD, Han J, Zhang J, Zhou C (2011) Blood–brain barrier disruption following subarchnoid hemorrhage may be faciliated through PUMA induction of endothelial cell apoptosis from the endoplasmic reticulum. Exp Neurol 230:240–247
Yatsushige H, Ostrowski RP, Tsubokawa T, Colohan A, Zhang JH (2007) Role of c-Jun N-terminal kinase in early brain injury after subarachnoid hemorrhage. J Neurosci Res 85:1436–1448
Yatsushige H, Yamaguchi-Okada M, Zhou C, Calvert JW, Cahill J, Colohan AR, Zhang JH (2008) Inhibition of c-Jun N-terminal kinase pathway attenuates cerebral vasospasm after experimental subarachnoid hemorrhage through the suppression of apoptosis. Acta Neurochir Suppl 104:27–31
Zhou Y, Martin RD, Zhang JH (2011) Advances in experimental subarachnoid hemorrhage. Acta Neurochir Suppl 110:15–21
Conflicts of interest
None.
Author information
Authors and Affiliations
Corresponding author
Additional information
Comment
The authors are investigating the mechanism underlying their previous observation that inhibition of JNK activation with SP600125 is neuroprotective following experimental rat SAH. The hypothesis is that administration of this agent preserves tight junctions at the capillary endothelium level and prevents BBB disruption following SAH.
The investigators’ approach is clever and multifaceted, looking at morphologic brain endpoints (edema, BBB disruption) and mechanistic endpoints (ultrastructure of the BBB, protein structure and regulation at the BBB). Consideration of the method shows that techniques are standard and the experimental observations are appropriately controlled. The SAH was created with a sharpened suture endovascular carotid perforation, and agent was administered pre- and post-injury. BBB permeability was evaluated with Evans blue. Selected protein analysis was with Western blot and apoptosis was measured with TUNEL counts. Cellular ultrastructure was assessed with TEM.
Experimental observations are both clinical and mechanistic. Clinically, SP600125 administration decreased Evans blue dye-observed BBB disruption. Brain water content (reflecting edema) was likewise statistically diminished in the treated group, and apoptosis was decreased as well. Mechanistically, by TEM there was more tight junction integrity in the treatment group. Integrity proteins at the BBB claudin-5 and ZO-1 were preserved by SP600125 dosing, while JNK1 and JNK3 were decreased. The implication is that JNK signals the deleterious BBB changes following experimental SAH.
As the authors conclude, targeting JNK signalling may be one way to increase neuroprotection and neural preservation following SAH. Certainly there are many inflection points in the SAH injury process. This excellent manuscript, by experienced and sophisticated investigators, rigorously follows one such thread, and as such adds value to our knowledge of SAH brain injury, although any clinical application, if appropriate, remains far away.
Christopher M. Loftus
Philadelphia, PA
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. S1
Determination of mortality rates 24 h after subarachnoid hemorrhage (SAH). SP(10), SP(30) treatment with 10 and 30 mg/kg SP600125, respectively. n.s. no significance (DOC 44 kb)
Rights and permissions
About this article
Cite this article
Chen, D., Wei, Xt., Guan, Jh. et al. Inhibition of c-Jun N-terminal kinase prevents blood–brain barrier disruption and normalizes the expression of tight junction proteins clautin-5 and ZO-1 in a rat model of subarachnoid hemorrhage. Acta Neurochir 154, 1469–1476 (2012). https://doi.org/10.1007/s00701-012-1328-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00701-012-1328-y