Abstract
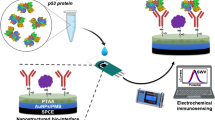
A label-free nanoimmunosensor is reported based on p53/CeO2/PEDOT nanobiocomposite-decorated screen-printed gold electrodes (SPAuE) for the electrochemical detection of anti-p53 autoantibodies. CeO2 nanoparticles (NPs) were synthesized and stabilized with cyanopropyltriethoxysilane by a soft chemistry method. The nanoimmunosensing architecture was prepared by in situ electropolymerization of 3,4-ethylenedioxythiophene (EDOT) on SPAuE in the presence of CeO2 NPs. The CeO2 NPs and Ce/PEDOT/SPAuE were characterized by scanning and transmission electron microscopy, dynamic and electrophoretic light scattering, ultraviolet–visible spectrophotometry, X-ray diffraction, Fourier-transform infrared spectroscopy, cyclic voltammetry, and electrochemical impedance spectroscopy. Ce/PEDOT/SPAuE was biofunctionalized with p53 antigen by covalent bonding for the label-free determination of anti-p53 autoantibodies by differential pulse voltammetry. The nanobiocomposite-based nanoimmunosensor detected anti-p53 autoantibodies in a linear range from 10 to 1000 pg mL−1, with a limit of detection (LOD) of 3.2 pg mL−1. The nanoimmunosensor offered high specificity, selectivity, and long-term storage stability with great potential to detect anti-p53 autoantibodies in serum samples. Overall, incorporating organo-functional nanoparticles into polymeric matrices can provide a simple-to-assemble, rapid, and ultrasensitive approach for on-site screening of anti-p53 autoantibodies and other disease-related biomarkers with low sample volumes.
Graphical abstract







Similar content being viewed by others
References
Tan HT, Low J, Lim SG, Chung MCM (2009) Serum autoantibodies as biomarkers for early cancer detection. FEBS J 276:6880–6904. https://doi.org/10.1111/j.1742-4658.2009.07396.x
Suppiah A, Greenman J (2013) Clinical utility of anti-p53 auto-antibody: systematic review and focus on colorectal cancer. World J Gastroenterol 19:4651–4670. https://doi.org/10.3748/wjg.v19.i29.4651
Portefaix J-M, Fanutti C, Granier C et al (2002) Detection of anti-p53 antibodies by ELISA using p53 synthetic or phage-displayed peptides. J Immunol Methods 259:65–75. https://doi.org/10.1016/S0022-1759(01)00494-X
Adeniyi O, Sicwetsha S, Adesina A, Mashazi P (2021) Immunoassay detection of tumor-associated autoantibodies using protein G bioconjugated to nanomagnet-silica decorated with Au@Pd nanoparticles. Talanta 226:122127. https://doi.org/10.1016/j.talanta.2021.122127
Adeniyi OK, Ngqinambi A, Mashazi PN (2020) Ultrasensitive detection of anti-p53 autoantibodies based on nanomagnetic capture and separation with fluorescent sensing nanobioprobe for signal amplification. Biosens Bioelectron 170:112640. https://doi.org/10.1016/j.bios.2020.112640
Lin Y-H, Wu C-C, Chen W-L, Chang K-P (2020) Anti-p53 autoantibody detection in automatic glass capillary immunoassay platform for screening of oral cavity squamous cell carcinoma. Sensors 20:971. https://doi.org/10.3390/s20040971
Elshafey R, Siaj M, Tavares AC (2016) Au nanoparticle decorated graphene nanosheets for electrochemical immunosensing of p53 antibodies for cancer prognosis. Analyst 141:2733–2740. https://doi.org/10.1039/c6an00044d
García-Miranda Ferrari A, Rowley-Neale SJ, Banks CE (2021) Screen-printed electrodes: transitioning the laboratory in-to-the field. Talanta Open 3:100032. https://doi.org/10.1016/j.talo.2021.100032
Sabaté del Río J, Henry OYF, Jolly P, Ingber DE (2019) An antifouling coating that enables affinity-based electrochemical biosensing in complex biological fluids. Nat Nanotechnol 14:1143–1149. https://doi.org/10.1038/s41565-019-0566-z
Fernández-Sánchez C, Pellicer E, Orozco J et al (2009) Plasma-activated multi-walled carbon nanotube–polystyrene composite substrates for biosensing. Nanotechnology 20:335501. https://doi.org/10.1088/0957-4484/20/33/335501
Mendoza E, Orozco J, Jiménez-Jorquera C et al (2008) Scalable fabrication of immunosensors based on carbon nanotube polymer composites. Nanotechnology 19:75102. https://doi.org/10.1088/0957-4484/19/7/075102
Cui M, Song Z, Wu Y et al (2016) A highly sensitive biosensor for tumor maker alpha fetoprotein based on poly(ethylene glycol) doped conducting polymer PEDOT. Biosens Bioelectron 79:736–741. https://doi.org/10.1016/j.bios.2016.01.012
Wang W, Han R, Chen M, Luo X (2021) Antifouling peptide hydrogel based electrochemical biosensors for highly sensitive detection of cancer biomarker HER2 in human serum. Anal Chem 93:7355–7361. https://doi.org/10.1021/acs.analchem.1c01350
Jolly P, Miodek A, Yang D-K et al (2016) Electro-engineered polymeric films for the development of sensitive aptasensors for prostate cancer marker detection. ACS Sensors 1:1308–1314. https://doi.org/10.1021/acssensors.6b00443
Aydemir N, Malmström J, Travas-Sejdic J (2016) Conducting polymer based electrochemical biosensors. Phys Chem Chem Phys 18:8264–8277. https://doi.org/10.1039/c5cp06830d
Fenoy GE, Azzaroni O, Knoll W, Marmisollé WA (2021) Functionalization strategies of PEDOT and PEDOT:PSS films for organic bioelectronics applications. Chemosensors 9:212. https://doi.org/10.3390/chemosensors9080212
Promsuwan K, Meng L, Suklim P et al (2020) Bio-PEDOT: modulating carboxyl moieties in poly(3,4-ethylenedioxythiophene) for enzyme-coupled bioelectronic interfaces. ACS Appl Mater Interfaces 12:39841–39849. https://doi.org/10.1021/acsami.0c10270
Wang W, Cui M, Song Z, Luo X (2016) An antifouling electrochemical immunosensor for carcinoembryonic antigen based on hyaluronic acid doped conducting polymer PEDOT. RSC Adv 6:88411–88416. https://doi.org/10.1039/c6ra19169j
Lin P, Chai F, Zhang R et al (2016) Electrochemical synthesis of poly(3,4-ethylenedioxythiophene) doped with gold nanoparticles, and its application to nitrite sensing. Microchim Acta 183:1235–1241. https://doi.org/10.1007/s00604-016-1751-5
Hartati YW, Letelay LK, Gaffar S et al (2020) Cerium oxide-monoclonal antibody bioconjugate for electrochemical immunosensing of HER2 as a breast cancer biomarker. Sens Bio-Sensing Res 27:100316. https://doi.org/10.1016/j.sbsr.2019.100316
Sappia LD, Piccinini E, Marmisollé W et al (2017) Integration of biorecognition elements on PEDOT platforms through supramolecular interactions. Adv Mater Interfaces 4:1–11. https://doi.org/10.1002/admi.201700502
Sonuç Karaboğa MN, Sezgintürk MK (2018) A novel silanization agent based single used biosensing system: detection of C-reactive protein as a potential Alzheimer’s disease blood biomarker. J Pharm Biomed Anal 154:227–235. https://doi.org/10.1016/j.jpba.2018.03.016
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Yu T, Zeng J, Lim B, **a Y (2010) Aqueous-phase synthesis of Pt/CeO2 hybrid nanostructures and their catalytic properties. Adv Mater 22:5188–5192. https://doi.org/10.1002/adma.201002763
Zhu J, Yang J, Xu Z et al (2017) Silicon anodes protected by a nitrogen-doped porous carbon shell for high-performance lithium-ion batteries. Nanoscale 9:8871–8878. https://doi.org/10.1039/c7nr01545c
Feyzi-barnaji B, Dinarvand R, Salehzadeh H et al (2021) Construction of a ternary nano-architecture based graphene oxide sheets, toward electrocatalytic determination of tumor-associated anti-p53 autoantibodies in human serum. Talanta 230:122276. https://doi.org/10.1016/j.talanta.2021.122276
Leo Di G, Sardanelli F (2020) Statistical significance: p value, 0.05 threshold, and applications to radiomics—reasons for a conservative approach. Eur Radiol Exp 4:18. https://doi.org/10.1186/s41747-020-0145-y
Stolarczyk JK, Ghosh S, Brougham DF (2009) Controlled growth of nanoparticle clusters through competitive stabilizer desorption. Angew Chemie - Int Ed 48:175–178. https://doi.org/10.1002/anie.200803895
Ghosh S, Divya D, Remani KC, Sreeremya TS (2010) Growth of monodisperse nanocrystals of cerium oxide during synthesis and annealing. J Nanoparticle Res 12:1905–1911. https://doi.org/10.1007/s11051-009-9753-4
Forge D, Laurent S, Gossuin Y et al (2011) An original route to stabilize and functionalize magnetite nanoparticles for theranosis applications. J Magn Magn Mater 323:410–415. https://doi.org/10.1016/j.jmmm.2010.09.031
Sifontes AB, Gonzalez G, Ochoa JL et al (2011) Chitosan as template for the synthesis of ceria nanoparticles. Mater Res Bull 46:1794–1799. https://doi.org/10.1016/j.materresbull.2011.07.049
Wang Y, Chen L, Liu Z et al (2018) A cationic surfactant-induced selective etching strategy for the synthesis of organosilica hollow nanospheres. J Sol-Gel Sci Technol 87:147–155. https://doi.org/10.1007/s10971-018-4705-z
Liu Z, Chen L, Ye X et al (2016) Selective basic etching of bifunctional core–shell composite particles for the fabrication of organic functionalized hollow mesoporous silica nanospheres. New J Chem 40:825–831. https://doi.org/10.1039/C5NJ02906F
Buśko-Oszczapowicz I, Oszczapowicz J (1991) Detection and determination of imidic acid derivatives. In: The Chemistry of Amidines and Related Compounds. Amidines and Imidates 2:231–299
Atta AM, Al-Lohedan HA, Al-Hussain SA (2015) Functionalization of magnetite nanoparticles as oil spill collector. Int J Mol Sci 16:6911–6931. https://doi.org/10.3390/ijms16046911
Kakhki S, Barsan MM, Shams E, Brett CMA (2012) Development and characterization of poly(3,4-ethylenedioxythiophene)-coated poly(methylene blue)-modified carbon electrodes. Synth Met 161:2718–2726. https://doi.org/10.1016/j.synthmet.2011.10.007
Moreira Gonçalves L (2021) Electropolymerized molecularly imprinted polymers: perceptions based on recent literature for soon-to-be world-class scientists. Curr Opin Electrochem 25:100640. https://doi.org/10.1016/j.coelec.2020.09.007
Melato AI, Mendonça MH, Abrantes LM (2009) Effect of the electropolymerisation conditions on the electrochemical, morphological and structural properties of PEDOTh films. J Solid State Electrochem 13:417–426. https://doi.org/10.1007/s10008-008-0522-6
Meng L, Turner APF, Mak WC (2019) Modulating electrode kinetics for discrimination of dopamine by a PEDOT:COOH interface doped with negatively charged tricarboxylate. ACS Appl Mater Interfaces 11:34497–34506. https://doi.org/10.1021/acsami.9b12946
Zhou Y, Wang Z, Yue W, et al (2009) Label-free detection of p53 antibody using a microcantilever biosensor with piezoresistive readout. In: SENSORS, 2009 IEEE Xplore. IEEE, Christchurch, pp 819–822
Adeniyi OK, Mashazi PN (2020) Stable thin films of human P53 antigen on gold surface for the detection of tumour associated anti-P53 autoantibodies. Electrochim Acta 331:135272. https://doi.org/10.1016/j.electacta.2019.135272
Danaei G, Finucane MM, Lu Y et al (2011) National, regional, and global trends in fasting plasma glucose and diabetes prevalence since 1980: systematic analysis of health examination surveys and epidemiological studies with 370 country-years and 2.7 million participants. Lancet 378:31–40. https://doi.org/10.1016/S0140-6736(11)60679-X
Hagel AF, Albrecht H, Dauth W et al (2017) Plasma concentrations of ascorbic acid in a cross section of the German population. J Int Med Res 46:168–174. https://doi.org/10.1177/0300060517714387
Goode JA, Rushworth JVH, Millner PA (2015) Biosensor regeneration: a review of common techniques and outcomes. Langmuir 31:6267–6276. https://doi.org/10.1021/la503533g
Soto D, Alzate M, Gallego J, Orozco J (2021) Hybrid nanomaterial/catalase-modified electrode for hydrogen peroxide sensing. J Electroanal Chem 880:114826. https://doi.org/10.1016/j.jelechem.2020.114826
Acknowledgements
We thank The Ruta N complex and EPM for hosting the Max Planck Tandem Groups.
Funding
The work has been funded by MINCIENCIAS, MINEDUCACIÓN, MINCIT, and ICETEX through the Program Ecosistema Científico Cod. FP44842-211–2018, project number 58536. J. O. received support from The University of Antioquia and the Max Planck Society through the cooperation agreement 566–1, 2014.
Author information
Authors and Affiliations
Contributions
Andrés F. Cruz-Pacheco: conceptualization; methodology; formal analysis; investigation; data curation; writing—original draft. Jennifer Quinchia: investigation; data curation; writing—original draft. Jahir Orozco: conceptualization; formal analysis; writing—review and editing; supervision; project administration; funding acquisition.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Cruz-Pacheco, A.F., Quinchia, J. & Orozco, J. Cerium oxide–doped PEDOT nanocomposite for label-free electrochemical immunosensing of anti-p53 autoantibodies. Microchim Acta 189, 228 (2022). https://doi.org/10.1007/s00604-022-05322-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-022-05322-5