Abstract
A novel polydopamine coated three-dimensional porous graphene aerogel sorbent carrying immobilized titanium(IV) ions (denoted as Ti4+@PDA@GA) was fabricated without using an organic solvent. The material is shown to be a viable carbon foam type of monolithic sorbent for selective lab-in-syringe enrichment of phosphoproteins and phosphopeptides. The phosphoproteins can be separated from a sample by aspiration and then bind to the sorbent. The analytes then can be dispensed within 5 min. The weight percent of titanium in the monolith typically is 14%, and the absorption capacities for the model proteins β-casein and κ-casein are 1300 and 1345 mg g−1, respectively. The absorption capacities for nonphosphoproteins are much smaller, typically 160 mg g−1 for β-lactoglobulin, 125 mg g−1 for bovine serum, and 4.8 mg g−1 for lysozyme. The results demonstrate that the selectivity for phosphoproteins was excellent on multiple biological samples including standard protein mixtures, spiked human blood serum, and drinking milk. The selective enrichment of phosphopeptides also makes the method a promising tool in phosphoproteomics.

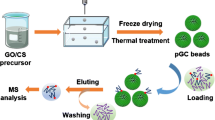
Schematic of a polydopamine coated three-dimensional porous graphene aerogel for immobilization of titanium(IV) ions. The material served as a monolithic sorbent for selective enrichment of phosphopeptides and phosphoproteins from biological samples. The enrichment process can be carried out conveniently using a lab-in-syringe way.








Similar content being viewed by others
References
Hunter T (2000) Signaling—2000 and beyond. Cell 100(1):113–127
Humphrey SJ, Azimifar SB, Mann M (2015) High-throughput phosphoproteomics reveals in vivo insulin signaling dynamics. Nat Biotechnol 33(9):990–995. https://doi.org/10.1038/nbt.3327
Tan CSH, Bodenmiller B, Pasculescu A, Jovanovic M, Hengartner MO, Jørgensen C, Bader GD, Aebersold R, Pawson T, Linding R (2009) Comparative analysis reveals conserved protein phosphorylation networks implicated in multiple diseases. Sci Signal 2 (81):ra39-ra39
Thingholm TE, Jensen ON, Larsen MR (2009) Analytical strategies for phosphoproteomics. Proteomics 9(6):1451–1468
Witze ES, Old WM, Resing KA, Ahn NG (2007) Map** protein post-translational modifications with mass spectrometry. Nat Methods 4(10):798–806
Li X-S, Yuan B-F, Feng Y-Q (2016) Recent advances in phosphopeptide enrichment: strategies and techniques. Trends Anal Chem 78:70–83. https://doi.org/10.1016/j.trac.2015.11.001
He XM, Chen X, Zhu GT, Wang Q, Yuan BF, Feng YQ (2015) Hydrophilic carboxyl cotton Chelator for titanium(IV) immobilization and its application as novel fibrous sorbent for rapid enrichment of Phosphopeptides. ACS Appl Mater Interfaces 7(31):17356–17362. https://doi.org/10.1021/acsami.5b04572
Ma X, Ding C, Yao X, Jia L (2016) Ethylene glycol assisted preparation of Ti4+-modified polydopamine coated magnetic particles with rough surface for capture of phosphorylated proteins. Anal Chim Acta 929:23–30. https://doi.org/10.1016/j.aca.2016.04.058
Yang X, **a Y (2016) Urea-modified metal-organic framework of type MIL-101(Cr) for the preconcentration of phosphorylated peptides. Microchim Acta 183(7):2235–2240. https://doi.org/10.1007/s00604-016-1860-1
Zhang H, Ou J, Yao Y, Wang H, Liu Z, Wei Y, Ye M (2017) Facile preparation of titanium(IV)-immobilized hierarchically porous hybrid monoliths. Anal Chem 89(8):4655–4662. https://doi.org/10.1021/acs.analchem.7b00242
Fei R, Zhang T, Huang Y, Hu Y (2017) Highly selective enrichment of phosphorylated proteins by using spore@ Fe3+ microspheres. Anal Chim Acta 986:161–170. https://doi.org/10.1016/j.aca.2017.07.030
**ong Z, Zhang L, Fang C, Zhang Q, Ji Y, Zhang Z, Zhang W, Zou H (2014) Ti4+-immobilized multilayer polysaccharide coated magnetic nanoparticles for highly selective enrichment of phosphopeptides. J Mater Chem B 2(28):4473–4480. https://doi.org/10.1039/c4tb00479e
Capriotti AL, Cavaliere C, Ferraris F, Gianotti V, Laus M, Piovesana S, Sparnacci K, Zenezini Chiozzi R, Lagana (2018) A new Ti-IMAC magnetic polymeric nanoparticles for phosphopeptide enrichment from complex real samples. Talanta 178:274–281
Kailasa SK, Wu HF (2014) Recent developments in nanoparticle-based MALDI mass spectrometric analysis of phosphoproteomes. Microchim Acta 181(9–10):853–864. https://doi.org/10.1007/s00604-014-1191-z
Ma W, Zhang F, Li L, Chen S, Qi L, Liu H, Bai Y (2016) Facile synthesis of Mesocrystalline SnO2 Nanorods on reduced graphene oxide sheets: an appealing multifunctional affinity probe for sequential enrichment of endogenous peptides and Phosphopeptides. ACS Appl Mater Interfaces 8(51):35099–35105. https://doi.org/10.1021/acsami.6b14597
S-f R, Y-l G (2006) Carbon nanotubes (2,5-dihydroxybenzoyl hydrazine) derivative as pH adjustable enriching reagent and matrix for MALDI analysis of trace peptides. J Am Soc Mass Spectrom 17(7):1023–1027
Qin H, Gao P, Wang F, Zhao L, Zhu J, Wang A, Zhang T, Wu Ra, Zou H (2011) Highly efficient extraction of serum peptides by ordered mesoporous carbon. Angew Chem Int Ed 50 (51):12218–12221
Zhang L, Gan Y, Sun H, Yu B, ** X, Zhang R, Zhang W, Zhang L (2017) Magnetic mesoporous carbon composites incorporating hydrophilic metallic nanoparticles for enrichment of phosphopeptides prior to their determination by MALDI-TOF mass spectrometry. Microchim Acta 184(2):547–555. https://doi.org/10.1007/s00604-016-2046-6
Matsuura K, Saito T, Okazaki T, Ohshima S, Yumura M, Iijima S (2006) Selectivity of water-soluble proteins in single-walled carbon nanotube dispersions. Chem Phys Lett 429(4):497–502
Han Q, Yang L, Liang Q, Ding M (2017) Three-dimensional hierarchical porous graphene aerogel for efficient adsorption and preconcentration of chemical warfare agents. Carbon 122:556–563
Han S, Wu D, Li S, Zhang F, Feng X (2014) Porous graphene materials for advanced electrochemical energy storage and conversion devices. Adv Mater 26(6):849–864
Uzzaman A, Shang Z, Qiao Z, Cao CX, **ao H (2018) Graphene and graphene oxide as a solid matrix for extraction of membrane and membrane-associated proteins. Microchim Acta 185(2):123. https://doi.org/10.1007/s00604-017-2658-5
Sun W, Cao L, Deng Y, Gong S, Shi F, Li G, Sun Z (2013) Direct electrochemistry with enhanced electrocatalytic activity of hemoglobin in hybrid modified electrodes composed of graphene and multi-walled carbon nanotubes. Anal Chim Acta 781:41–47
Wan W, Han Q, Zhang X, **e Y, Sun J, Ding M (2015) Selective enrichment of proteins for MALDI-TOF MS analysis based on molecular imprinting. Chem Commun 51(17):3541–3544. https://doi.org/10.1039/c4cc10205c
Yan Y, Zheng Z, Deng C, Li Y, Zhang X, Yang P (2013) Hydrophilic polydopamine-coated graphene for metal ion immobilization as a novel immobilized metal ion affinity chromatography platform for phosphoproteome analysis. Anal Chem 85(18):8483–8487. https://doi.org/10.1021/ac401668e
Gao H, Sun Y, Zhou J, Xu R, Duan H (2013) Mussel-inspired synthesis of polydopamine-functionalized graphene hydrogel as reusable adsorbents for water purification. ACS Appl Mater Interfaces 5(2):425–432
Wang X, Deng C (2015) Preparation of magnetic graphene@polydopamine@Zr-MOF material for the extraction and analysis of bisphenols in water samples. Talanta 144:1329–1335
Lee H, Dellatore SM, Miller WM, Messersmith PB (2007) Mussel-inspired surface chemistry for multifunctional coatings. Science 318(5849):426–430
Moser J, Punchihewa S, Infelta PP, Graetzel M (1991) Surface complexation of colloidal semiconductors strongly enhances interfacial electron-transfer rates. Langmuir 7(12):3012–3018
Zhou H, Ye M, Dong J, Corradini E, Cristobal A, Heck AJ, Zou H, Mohammed S (2013) Robust phosphoproteome enrichment using monodisperse microsphere-based immobilized titanium(IV) ion affinity chromatography. Nat Protoc 8(3):461–480
Han Q, Liang Q, Zhang X, Yang L, Ding M (2016) Graphene aerogel based monolith for effective solid-phase extraction of trace environmental pollutants from water samples. J Chromatogr A 1447:39–46. https://doi.org/10.1016/j.chroma.2016.04.032
Liu W, Zheng J, Li S, Wang R, Lin Z, Yang H (2015) Aluminium glycinate functionalized silica nanoparticles for highly specific separation of phosphoproteins. J Mater Chem B 3(31):6528–6535. https://doi.org/10.1039/c5tb01055a
Deng Q, Wu J, Chen Y, Zhang Z, Wang Y, Fang G, Wang S, Zhang Y (2014) Guanidinium functionalized superparamagnetic silica spheres for selective enrichment of phosphopeptides and intact phosphoproteins from complex mixtures. J Mater Chem B 2(8):1048–1058. https://doi.org/10.1039/c3tb21540g
Ma X, Jia L (2016) Polydopamine assisted preparation of Ti4+-decorated magnetic particles for selective and rapid adsorption of phosphorylated proteins. J Chem Technol Biot 91(4):892–900. https://doi.org/10.1002/jctb.4654
Cheng G, Wang ZG, Liu YL, Zhang JL, Sun DH, Ni JZ (2013) Magnetic affinity microspheres with meso−/macroporous shells for selective enrichment and fast separation of phosphorylated biomolecules. ACS Appl Mater Interfaces 5(8):3182–3190
Acknowledgements
This work was supported by the Natural Science Foundation of China [grant numbers 21575076, 21621003]; the National Key Research and Development Program of China [grant numbers 2016YFA0203101,2017YFC0906902]; and the Bei**g Municipality Science and Technology Program [grant numbers D161100002116001].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1.39 mb)
Rights and permissions
About this article
Cite this article
Tan, S., Wang, J., Han, Q. et al. A porous graphene sorbent coated with titanium(IV)-functionalized polydopamine for selective lab-in-syringe extraction of phosphoproteins and phosphopeptides. Microchim Acta 185, 316 (2018). https://doi.org/10.1007/s00604-018-2846-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-018-2846-y