Abstract
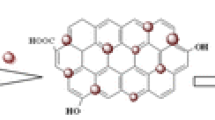
Graphene oxide (GO) was conjugated to magnetite nanoparticles and then coated with poly(methyl methacrylate) to obtain a nanomaterial of the type PMMA@GO-Fe3O4, which is shown to be a viable sorbent for magnetic solid-phase extraction. The nanocomposite was characterized by SEM, TEM, XRD, VSM and FTIR spectroscopy. It was applied to the extraction of the aromatic amines aniline, N,N-dimethylaniline, o-toluidine, and 3-chloroaniline. Under optimal conditions, the preconcentration factors range from 139 to 173. Following desorption with dichloromethane, the amines were quantified by GC. Analytical figures of merit include (a) a linear range extending from 0.007–100 ng mL−1, (b) detection limits between 2 and 6 pg mL−1; and (c) relative standard deviations between 5.9 and 8.6% (for n = 5; at 0.05, 5.0 and 50 ng mL−1 levels, respectively). The method was successfully applied to the determination of aromatic amines in spiked real water samples and gave recoveries in the range of 90.3%–99.0%.

A magnetic sorbent was fabricated by deposition of poly(methyl methacrylate) onto Fe3O4-magnetized graphene oxide. It was applied to the extraction and preconcentration of aromatic amines from water samples prior to their gas chromatography–flame ionization detector (GC–FID) determination.





Similar content being viewed by others
References
Jurado-Sánchez B, Ballesteros E, Gallego M (2011) Gas chromatographic determination of N-nitrosamines, aromatic amines, and melamine in milk and dairy products using an automatic solid-phase extraction system. J Agric Food Chem 59:7519–7526
Sun Y, Liang L, Zhao X, Yu L, Zhang J, Shi G, Zhou T (2009) Determination of aromatic amines in water samples by capillary electrophoresis with amperometric detection. Water Res 43:41–46
Tan C, Wang Y, Deng Z, Xu N, Song X, Liu H, Rong M, Chen X (2014) A nanocomposite disk prepared from reduced graphene oxide and gold nanoparticles for the preconcentration of heterocyclic aromatic amines prior to their determination by HPLC. Microchim Acta 181:821–828
Zhou Q, Ye C (2008) Ionic liquid for improved single-drop microextraction of aromatic amines in water samples. Microchim Acta 162:153–159
Jalilian N, Ebrahimzadeh H, Asgharinezhad AA (2017) Dispersive micro-solid phase extraction of aromatic amines based on an efficient sorbent made from poly(1,8-diaminonaphtalen) and magnetic multiwalled carbon nanotubes composite. J Chromatogr A 1499:38–47
Sarafraz-Yazdi A, Ardaki MS, Amiri A (2013) Determination of monocyclic aromatic amines using headspace solid-phase microextraction based on sol–gel technique prior to GC. J Sep Sci 36:1629–1635
Amiri A, Baghayeri M, Kashmari M (2016) Magnetic nanoparticles modified with polyfuran for the extraction of polycyclic aromatic hydrocarbons prior to their determination by gas chromatography. Microchim Acta 183:149–156
Amiri A, Baghayeri M, Nori S (2015) Magnetic solid-phase extraction using poly(para-phenylenediamine) modified with magnetic nanoparticles as adsorbent for analysis of monocyclic aromatic amines in water and urine samples. J Chromatogr A 1415:20–26
Amiri A, Saadati-Moshtaghin HR, Zonoz FM, Targhoo A (2017) Preparation and characterization of magnetic wells–Dawson heteropoly acid nanoparticles for magnetic solid-phase extraction of aromatic amines in water samples. J Chromatogr A 1483:64–70
Han D, Yan H, Row KH (2011) Ionic liquid-based dispersive liquid–liquid microextraction for sensitive determination of aromatic amines in environmental water. J Sep Sci 34:1184–1189
Vasconcelos I, Fernandes C (2017) Magnetic solid phase extraction for determination of drugs in biological matrices. Trends Anal Chem 89:41–52
Zhang W, Yan Z, Gao J, Tong P, Liu W, Zhang L (2015) Metal–organic framework UiO-66 modified magnetite@silica core–shell magnetic microspheres for magnetic solid-phase extraction of domoic acid from shellfish samples. J Chromatogr A 1400:10–18
Safari M, Yamini Y, Masoomi MY, Morsali A, Mani-Varnosfaderani A (2017) Magnetic metal-organic frameworks for the extraction of trace amounts of heavy metal ions prior to their determination by ICP-AES. Microchim Acta 184:1555–1564
Bagheri H, Roostaie A, Baktash MY (2014) A chitosan–polypyrrole magnetic nanocomposite as μ-sorbent for isolation of naproxen. Anal Chim Acta 816:1–7
Tahmasebi E, Yamini Y (2014) Polythiophene-coated Fe3O4 nanoparticles as a selective adsorbent for magnetic solid-phase extraction of silver(I), gold(III), copper(II) and palladium(II). Microchim Acta 181:543–551
Qin X, AbdAlkhalig A, Bakheet A, Zhu X (2017) Fe3O4@ionic liquid-β-cyclodextrin polymer magnetic solid phase extraction coupled with HPLC for the separation/analysis of congo red. J Iran Chem Soc 14:2017–2022
Cai Q, Yang Z, Chen N, Zhou X, Hong J (2015) Selective capture and rapid identification of Panax notoginseng metabolites in rat faeces by the integration of magnetic molecularly imprinted polymers and high-performance liquid chromatography coupled with orbitrap mass spectrometry. J Chromatogr A 1455:65–73
Moreno V, Zougagh M, Ríos Á (2016) Hybrid nanoparticles based on magnetic multiwalled carbon nanotube-nanoC18SiO2 composites for solid phase extraction of mycotoxins prior to their determination by LC-MS. Microchim Acta 183:871–880
Abbasi S, Sarafraz-Yazdi A, Amiri A, Ghaemi F (2017) Development of novel magnetic solid-phase extraction sorbent based on Fe3O4/carbon nanosphere/polypyrrole composite and their application to the enrichment of polycyclic aromatic hydrocarbons from water samples prior to GC–FID analysis. J Iran Chem Soc In Press. https://doi.org/10.1007/s13738-017-1218-6
Rezvani-Eivari M, Amiri A, Baghayeri M, Ghaemi F (2016) Magnetized graphene layers synthesized on the carbon nanofibers asnovel adsorbent for the extraction of polycyclic aromatic hydrocarbons from environmental water samples. J Chromatogr A 1465:1–8
Jalilian N, Ebrahimzadeh H, Asgharinezhad AK, Molaei K (2017) Extraction and determination of trace amounts of gold(III), palladium(II), platinum(II) and silver(I) with the aid of a magnetic nanosorbent made from Fe3O4-decorated and silica-coated graphene oxide modified with a polypyrrole-polythiophene copolymer. Microchim Acta 184:2191–2200
Asgharinezhad AK, Ebrahimzadeh H (2016) Poly(2-aminobenzothiazole)-coated graphene oxide/magnetite nanoparticles composite as an efficient sorbent for determination of non-steroidal anti-inflammatory drugs in urine sample. J Chromatogr A 1435:18–29
Asgharinezhad AK, Ebrahimzadeh H (2015) Coextraction of acidic, basic and amphiprotic pollutants using multiwalled carbon nanotubes/magnetite nanoparticles@polypyrrole composite. J Chromatogr A 1412:1–11
Wang X, Liu B, Lu Q, Qu Q (2014) Graphene-based materials: fabrication and application for adsorption in analytical chemistry. J Chromatogr A 1362:1–15
Su S, Chen B, He M, Hu B, **ao Z (2014) Determination of trace/ultratrace rare earth elements in environmental samples by ICP-MS after magnetic solid phase extraction with Fe3O4@SiO2@polyaniline–graphene oxide composite. Talanta 119:458–466
Mehdinia A, Khodaee N, Jabbari A (2015) Fabrication of graphene/Fe3O4@polythiophene nanocomposite and its application in the magnetic solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. Anal Chim Acta 868:1–9
Miao J, Zhang F, Takieddin M, Mousa S, Linhardt RJ (2012) Adsorption of doxorubicin on poly(methyl methacrylate)−chitosan−heparin-coated activated carbon beads. Langmuir 28:4396–4403
Mittal A, Ahmad R, Hasan I (2016) Poly(methyl methacrylate)-grafted alginate/Fe3O4 nanocomposite: synthesis and its application for the removal of heavy metal ions. Desalin Water Treat 57:19820–19833
Marcano DC, Kosynkin DV, Berlin JM, Sinitskii A, Sun Z, Slesarev A, Alemany LB, Lu W, Tour JM (2010) Improved synthesis of Graphene oxide. ACS Nano 4:4806–4814
Pham VH, Dang TT, Hur SH, Kim EJ, Chung JS (2012) Highly conductive poly(methyl methacrylate) (PMMA)-reduced Graphene oxide composite prepared by self-assembly of PMMA latex and Graphene oxide through electrostatic interaction. ACS Appl Mater Interfaces 4:2630–2636
Ali U, Karim KJBA, Buang NA (2015) A review of the properties and applications of poly (methyl methacrylate) (PMMA). Polym Rev 55:678–705
Ai Y, Zhao F, Zeng B (2015) Novel proton-type ionic liquid doped polyaniline for the headspace solid-phase microextraction of amines. Anal Chim Acta 880:60–66
Zeng Z, Qiu W, Yang M, Wei X, Huang Z, Li F (2001) Solid-phase microextraction of monocyclic aromatic amines using novel fibers coated with crown ether. J Chromatogr A 934:51–57
Minjia H, Chao T, Qunfang Z, Guibin J (2004) Preparation of polyaniline coating on a stainless-steel wire using electroplating and its application to the determination of six aromatic amines using headspace solid-phase microextraction. J Chromatogr A 1048:257–262
Reddy-Noone K, Jain A, Verma KK (2007) Liquid-phase microextraction and GC for the determination of primary, secondary and tertiary aromatic amines as their iodo-derivatives. Talanta 73:684–691
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Electronic supplementary material
ESM 1
(DOCX 1289 kb)
Rights and permissions
About this article
Cite this article
Bashtani, E., Amiri, A. & Baghayeri, M. A nanocomposite consisting of poly(methyl methacrylate), graphene oxide and Fe3O4 nanoparticles as a sorbent for magnetic solid-phase extraction of aromatic amines. Microchim Acta 185, 14 (2018). https://doi.org/10.1007/s00604-017-2587-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2587-3