Abstract
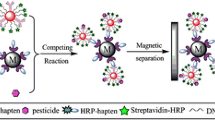
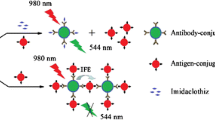
A new kind of labelled antibody was synthesized by modifying gold nanoparticles (AuNPs) with the fluorophore rhodamine B isothiocyanate (RBITC) and a secondary antibody (IgG). The conjugate thus obtained was used in a competitive sandwich assay with a turn-on signal change. It was designed to detect the organophosphorus pesticide chlorpyrifos. The fluorescence of the RBITC-labeled gold immunoprobe with emission at 575 nm and excitation at 556 nm is almost completely quenched. If, however, cysteamine is added, the fluorophore is released from the labeled secondary antibody and fluorescence increases in accordance with the quantity of secondary antibody bound to the sandwich. This assay was applied to determine chlorpyrifos in dried tangerine peels. The detection results were also independently confirmed by LC-MS/MS. The method allows the concentrations of chlorpyrifos to be quantified down to 4.9 ng·mL−1, which is equivalent to 61 μg·kg−1 in dried tangerine peels. In our perception, this approach has a wide potential to be applied in the determination of numerous analytes for which antibodies are available.

A new kind of labelled antibody was synthesized by modifying gold nanoparticles with fluorophore rhodamine B isothiocyanate (RBITC) and a secondary antibody (IgG). The conjugate was used in fluoroimmunoassay with a turn-on signal change. The method was designed to detect the organophosphorus pesticide chlorpyrifos.





Similar content being viewed by others
References
Nakano VE, Kussumi TA, Lemes VRR, Kimura IA, Rocha SB, Alaburda J, Oliveira MCC, Ribeiro RA, Faria ALR, Waldhelm KC (2016) Evaluation of pesticide residues in oranges from São Paulo. Brazil Food Sci Technol (Campinas) 36:40–48
Liu Y, Li S, Ni Z, Qu M, Zhong D, Ye C, Tang F (2016) Pesticides in persimmons, jujubes and soil from China: residue levels, risk assessment and relationship between fruits and soils. Sci Total Environ 542:620–628
Sadowska-Rociek A, Surma M, Cieślik E (2013) Application of QuEChERS method for simultaneous determination of pesticide residues and PAHs in fresh herbs. Bull Environ Contam Toxicol 90(4):508–513
Aragay G, Pino F, Merkoçi A (2012) Nanomaterials for sensing and destroying pesticides. Chem Rev 112(10):5317–5338
Arduini F, Cinti S, Scognamiglio V, Moscone D (2016) Nanomaterials in electrochemical biosensors for pesticide detection: advances and challenges in food analysis. Microchim Acta 183(7):2063–2083
Wang X, Cao Y, Chen D, Zhao G, Sun X (2014) An amperometric immunosensor based on graphene composite film and protein a for chlorpyrifos detection. Sensors Trans 178(9):47–55
Sun, Z, Wang, W, Wen, H, Gan, C, Lei, H and Liu, Y (2015) Sensitive electrochemical immunoassay for chlorpyrifos by using flake-like Fe3O4 modified carbon nanotubes as the enhanced multienzyme label. Anal Chim Acta 899 (Supplement C): 91–99
Xu ZL, Wang Q, Lei HT, Eremin SA, Shen YD, Wang H, Beier RC, Yang JY, Maksimova KA, Sun YM (2011) A simple, rapid and high-throughput fluorescence polarization immunoassay for simultaneous detection of organophosphorus pesticides in vegetable and environmental water samples. Anal Chim Acta 708(1–2):123–129
Beloglazova NV, Speranskaya ES, Wu A, Wang Z, Sanders M, Goftman VV, Zhang D, Goryacheva IY, De Saeger S (2014) Novel multiplex fluorescent immunoassays based on quantum dot nanolabels for mycotoxins determination. Biosens Bioelectron 62:59–65
Goldman, ER, Anderson, GP, Lebedev, N, Lingerfelt, BM, Winter, PT, Patterson, CH, Jr. and Mauro, JM (2003) Analysis of aqueous 2,4,6-trinitrotoluene (TNT) using a fluorescent displacement immunoassay. Anal Bioanal Chem 375(4): 471–475
Petrou PS, Mastichiadis C, Christofidis I, Kakabakos SE (2007) Glycerin suppression of fluorescence self-quenching and improvement of heterogeneous Fluoroimmunoassay sensitivity. Anal Chem 79(2):647–653
Tang D, Yu Y, Niessner R, Miro M, Knopp D (2010) Magnetic bead-based fluorescence immunoassay for aflatoxin B1 in food using biofunctionalized rhodamine B-doped silica nanoparticles. Analyst 135(10):2661–2667
Feng J, Shan G, Maquieira A, Koivunen ME, Guo B, Hammock BD, Kennedy IM (2003) Functionalized europium oxide nanoparticles used as a fluorescent label in an immunoassay for atrazine. Anal Chem 75(19):5282–5286
Viger ML, Live LS, Therrien OD, Boudreau D (2008) Reduction of self-quenching in fluorescent silica-coated silver nanoparticles. Plasmonics 3(1):33–40
Sharma P, Gandhi S, Chopra A, Sekar N, Raman Suri C (2010) Fluoroimmunoassay based on suppression of fluorescence self-quenching for ultra-sensitive detection of herbicide diuron. Anal Chim Acta 676(1–2):87–92
Wang Z, Heon Lee J, Lu Y (2008) Highly sensitive "turn-on" fluorescent sensor for Hg2+ in aqueous solution based on structure-switching DNA. Chem Commun (Camb) 45:6005–6007
Lin B, Yu Y, Li R, Cao Y, Guo M (2016) Turn-on sensor for quantification and imaging of acetamiprid residues based on quantum dots functionalized with aptamer. Sensors Actuators B Chem 229:100–109
Zhang J, Wang J, Yang L, Liu B, Guan G, Jiang C, Zhang Z (2014) Ligand replacement induced chemiluminescence for selective detection of an organophosphorus pesticide using bifunctional au-Fe3O4 dumbbell-like nanoparticles. Chem Commun 50(100):15870–15873
Zhang K, Mei Q, Guan G, Liu B, Wang S, Zhang Z (2010) Ligand replacement-induced fluorescence switch of quantum dots for ultrasensitive detection of Organophosphorothioate pesticides. Anal Chem 82(22):9579–9586
Wang Q, ** Y, Fu X, Ma M, Cai Z (2016) A "turn-on-off-on" fluorescence switch based on quantum dots and gold nanoparticles for discriminative detection of ovotransferrin. Talanta 150:407–414
Zhu J, Chang H, Li JJ, Li X, Zhao JW (2017) Dual-mode melamine detection based on gold nanoparticles aggregation-induced fluorescence “turn-on” and “turn-off” of CdTe quantum dots. Sensors Actuators B Chem 239:906–915
Maxwell DJ, Taylor JR, Nie S (2002) Self-assembled nanoparticle probes for recognition and detection of biomolecules. J Am Chem Soc 124(32):9606–9612
Dubertret B, Calame M, Libchaber AJ (2001) Single-mismatch detection using gold-quenched fluorescent oligonucleotides. Nat Biotechnol 19(4):365–370
Shang L, ** L, Dong S (2009) Sensitive turn-on fluorescent detection of cyanide based on the dissolution of fluorophore functionalized gold nanoparticles. Chem Commun (Camb) 21:3077–3079
Liu D, Wang S, Swierczewska M, Huang X, Bhirde AA, Sun J, Wang Z, Yang M, Jiang X, Chen X (2012) Highly robust, recyclable displacement assay for mercuric ions in aqueous solutions and living cells. ACS Nano 6(12):10999–11008
Dou X, Chu X, Kong W, Yang Y, Yang M (2015) Carbon nanotube-based QuEChERS extraction and enhanced product ion scan-assisted confirmation of multi-pesticide residue in dried tangerine peel. RSC Adv 5(105):86163–86171
Wang, J, Li, H, Zou, H, Wang, C, Zhang, H, Mano, JF and Song, W (2017) Flexible method for fabricating protein patterns on superhydrophobic platforms controlled by magnetic field. Biomater Sci 5(3):408–411
Shahabi S, Treccani L, Dringen R, Rezwan K (2015) Dual fluorophore doped silica nanoparticles for cellular localization studies in multiple stained cells. Acta Biomater 14:208–216
Qian X, Emory SR, Nie S (2012) Anchoring molecular Chromophores to colloidal gold Nanocrystals: surface-enhanced Raman evidence for strong electronic coupling and irreversible structural locking. J Am Chem Soc 134(4):2000–2003
Qian X, Peng X-H, Ansari DO, Yin-Goen Q, Chen GZ, Shin DM, Yang L, Young AN, Wang MD, Nie S (2008) In vivo tumor targeting and spectroscopic detection with surface-enhanced Raman nanoparticle tags. Nat Biotechnol 26(1):83–90
Liu D, Huang X, Wang Z, ** A, Sun X, Zhu L, Wang F, Ma Y, Niu G, Hight Walker AR, Chen X (2013) Gold nanoparticle-based Activatable probe for sensing ultralow levels of prostate-specific antigen. ACS Nano 7(6):5568–5576
Manju S, Sreenivasan K (2011) Detection of glucose in synthetic tear fluid using dually functionalized gold nanoparticles. Talanta 85(5):2643–2649
Ray PC, Darbha GK, Ray A, Walker J, Hardy W (2007) Gold nanoparticle based FRET for DNA detection. Plasmonics 2(4):173–183
Angioni A, Dedola F, Garau A, Sarais G, Cabras P, Caboni P (2011) Chlorpyrifos residues levels in fruits and vegetables after field treatment. J Environ Sci Health B 46(6):544–549
Golge O, Kabak B (2015) Determination of 115 pesticide residues in oranges by high-performance liquid chromatography–triple-quadrupole mass spectrometry in combination with QuEChERS method. J Food Compos Anal 41:86–97
Acknowledgements
The work was supported by National Natural Science Foundation of China (81573595, 81703699), National Project for Standardization of Chinese Materia Medica (ZYBZH-Y-JIN-34), CAMS Innovation Fund for Medical Sciences (2016-I2M-1-012, 2016-I2M-3-010, 2017-I2M-1-013).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The author(s) declare that they have no competing interests.
Additional information
This manuscript has been thoroughly edited by a native English speaker from an editing company.
Electronic supplementary material
ESM 1
(DOCX 2108 kb)
Rights and permissions
About this article
Cite this article
Dou, X., Zhang, L., Liu, C. et al. Fluorometric competitive immunoassay for chlorpyrifos using rhodamine-modified gold nanoparticles as a label. Microchim Acta 185, 41 (2018). https://doi.org/10.1007/s00604-017-2561-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00604-017-2561-0