Abstract
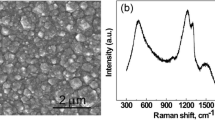
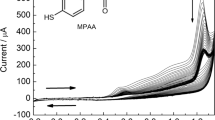
A sensitive tyrosinase biosensor, based on co-modifying tyrosinase and palygorskite on glassy carbon electrode, was developed for phenol analysis. Palygorskite, a kind of natural one-dimensional clay with good biocompatibility, high specific surface area and porous morphology, works as a perfect matrix of enzyme. Tyrosinase retains its inherent bioactivity when immobilized in palygorskite, which leads to a high sensitivity of 1.897 A mol−1 L. The sensor response achieves 95% of steady-state-current in no more than 3 s, and the linear range of the bioelectrode spans the concentration of phenol from 5 × 10−8 to 1 × 10−4 mol L−1 with a correlation coefficient of 0.9992. The results show no apparent decrease in the response over 2 weeks, and about 80% of the response was retained after 2 months when the electrode was stored at 4–5 °C.






Similar content being viewed by others
References
Yong G, Leone C, Strothkamp KG (1990) Agaricus bisporus metapotyrosinase: preparation, characterization, and conversion to mixed-metal derivatives of the binuclear site. Biochemistry 29:9684
Seo S, Sharma VK, Sharma N (2003) Mushroom tyrosinase: recent prospects. J Agric Food Chem 51:2837
May SW (1999) Applications of oxidoreductases. Biotechnol 10:370
Durán N, Esposito E (2000) Potential applications of oxidative enzymes and phenoloxidase-like compounds in wastewater and soil treatment: a review. Appl Catal B Environ 28:83
Castillo M, Domingues R et al (1997) Persistence of selected pesticides and their phenolic transformation products in natural waters using off-line liquid solid extraction followed by liquid chromatographic techniques. Anal Chim Acta 353:133
MacCrehan WA, Brown-Thomas JM (1987) Determination of phenols in petroleum crude oils using liquid chromatography with electrochemical detection. Anal Chem 59:477
Berger TA, Daye JF (1991) Separation of phenols by packed column supercritical fluid chromatography. J Chromatogr Sci 29:54
Brage C, Sjöström K (1991) Separation of phenols and aromatic hydrocarbons from biomass tar using aminopropylsilane normal-phase liquid chromatography. J Chromatogr 538:303
King WP, Joseph KT, Kissinger PT (1980) Liquid chromatography with amperometric detection for determining phenolic preservatives. J Assoc Anal Chem 63:137
Atlow SC, Bonadonna-Apro L, Klibanov AM (1984) Dephenolization of industrial wastewaters catalyzed by polyphenol oxidase. Biotech Bioeng 26:599
Vincent G, Angeletti G, Bjorseth A (eds) (1991) Organic micropollutants in the aquatic environment. Kluwer Academic, Dordrecht
Drinking Water Regulations and Health Advisories (1996) US Environmental Protection Agency, Office of Water, Washington, DC, EPA 822-B-96-002
Streffer K, Kaatz H et al (1998) Application of a sensitive catechol detector for determination of tyrosinase inhibitors. Anal Chim Acta 362:81
Huang TH, Kuwana T, Warsinke A (2002) Analysis of thiols with tyrosinase-modified carbon paste electrodes based on blocking of substrate recycling. Biosens Bioelectron 17:1107
Streffer K, Katz H et al (1998) Application of a sensitive catechol detector for determination of tyrosinase inhibitors. Anal Chim Acta 362:81
Kubo I, Kinst-Hori I et al (2000) Flavonols from Heterotheca inuloides: tyrosinase inhibitory activity and structural criteria. Bioorg Med Chem 8:1749
Serra B, Morales MD et al (2005) In-a-day electrochemical detection of coliforms in drinking water using a tyrosinase composite biosensor. Anal Biochem 336:8115
Wang J, Chen L (1995) Hydrazine detection using a tyrosinase-based inhibition biosensor. Anal Chem 67:3824
Yildiz HB, Castillo J et al (2007) Phenol biosensor based on electrochemically controlled integration of tyrosinase in a redox polymer. Microchimica Acta 159:27
Ahuja T, Mir IA, Kumar D (2007) Biomolecular immobilization on conducting polymers for biosensing applications. Biomaterials 28:791
Yu JH, Ju HX (2004) Pure organic phase phenol biosensor based on tyrosinase entrapped in a vapor deposited titania sol–gel membrane. Electroanalysis 16:1305
Liu ZJ, Liu BH et al (2000) Probing trace phenols based on mediator-free alumina sol–gel-derived tyrosinase biosensor. Anal Chem 72:4707
Wang B, Zhang J, Dong S (2000) Silica sol–gel composite film as an encapsulation matrix for the construction of an amperometric tyrosinase-based biosensor. Biosens Bioelectron 15:397
Dempsey E, Diamond D, Collier A (2004) Development of a biosensor for endocrine disrupting compounds based on tyrosinase entrapped within a poly(thionine) film. Biosens Bioelectron 20:367
Mailley P, Cummings EA et al (2003) Composite carbon paste biosensor for phenolic derivatives based on in situ electrogenerated polypyrrole binder. Anal Chem 75:5422
Chen J, Yu ZG et al (2008) Preparation of biofilm electrode with Xanthomonas sp. and carbon nanotubes and the application to rapid biochemical oxygen demand analysis in high-salt condition. Water Environ Res 80:699
Bradley WF (1940) The structural scheme of attapulgite. Am Mineral 25:405
Miao SD, Liu ZM et al (2007) Ionic liquid-assisted immobilization of Rh on attapulgite and its application in cyclohexene hydrogenation. J Phys Chem C 111:2185
Huang JH, Liu YF, Wang XG (2008) Influence of differently modified palygorskites in the immobilization of a lipase. J Mol Catal B 55:49
The united states pharmacopoeis 30th ed, (2008) the united states pharmacopoeial convention
Xu JM, Li W et al (2007) Direct electrochemistry of Cytochrome c on natural nano-attapulgite clay, modified electrode and its electrocatalytic reduction for H2O2. Electrochim Acta 52:3601
Cummings EA, Linquette-Mailley S et al (2001) A comparison of amperometric screen-printed, carbon electrodes and their application to the analysis of phenolic compounds present in beers. Talanta 55:1015
Bard AJ, Faulkner LR (2001) Electrochemical methods, fundamental and applications, 2nd edn. Wiley, New York
Kamin RA, Wilson GS (1980) Rotating ring-disk enzyme electrode for biocatalysis kinetic studies and characterization of the immobilized enzyme layer. Anal Chem 52:1198
Fenool LG, Rodríguez-López JN et al (2002) Michaelis constants of mushroom tyrosinase with respect to oxygen in the presence of monophenols and diphenols. Int J Biochem Cell Biol 34:332
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chen, J., **, Y. Sensitive phenol determination based on co-modifying tyrosinase and palygorskite on glassy carbon electrode. Microchim Acta 169, 249–254 (2010). https://doi.org/10.1007/s00604-010-0320-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00604-010-0320-6