Abstract
Purpose
To explore the role of indocyanine green (ICG) fluorescence navigation in laparoscopic hepatectomy and investigate if the timing of its administration influences the intraoperative observation.
Methods
The subjects of this retrospective study were 120 patients who underwent laparoscopic hepatectomy; divided into an ICG-FN group (n = 57) and a non-ICG-FN group (n = 63). We analyzed the baseline data and operative data.
Results
There were no remarkable differences in baseline data such as demographic characteristics, lesion-related characteristics, and liver function parameters between the groups. Operative time and intraoperative blood loss were significantly lower in the ICG-FN group. The rate of R0 resection of malignant tumors was comparable in the ICG-FN and non-ICG-FN groups, but the wide surgical margin rate was significantly higher in the ICG-FN group. The administration of ICG 0–3 or 4–7 days preoperatively did not affect the intraoperative fluorescence imaging. Operative time, intraoperative blood loss, and a wide surgical margin correlated with ICG fluorescence navigation. ICG fluorescence navigation helped to minimize intraoperative blood loss and achieve a wide surgical margin.
Conclusion
ICG fluorescence navigation is safe and efficient in laparoscopic hepatectomy. It helps to achieve a wide surgical margin, which could result in a better prognosis. The administration of ICG 0–3 days preoperatively is acceptable.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Laparoscopic hepatectomy is now performed widely to cure benign and malignant liver diseases [1]. To mark the demarcation line, portal staining or inflow clam** of the target area is recommended in conventional open anatomical liver resection [2,3,4]. Conversely, in laparoscopic hepatectomy, this requires advanced skills. The lack of tactile perception of laparoscopic forceps and the complexity of intraoperative ultrasound examination makes it challenging to localize the tumor and confirm the demarcation line, limiting the application of this technology [5].
Indocyanine green (ICG), once bound to protein, can emit fluorescence (peaking at 840 nm) under the illumination of near-infrared light [6]. Because it can be absorbed exclusively by hepatocytes and excreted through bile without enterohepatic recirculation, ICG has gain the attention of hepatobiliary surgeons over the last two decades [7]. Intraoperative ICG fluorescence navigation might be a complementary solution to overcome the limitation of laparoscopic hepatectomy [4, 8]. Its application can also improve the detection rate of liver tumor focus, especially small lesions in an early stage [9,10,11]. Moreover, laparoscopic hepatectomy using ICG fluorescence navigation is associated with less intraoperative blood loss, a lower transfusion rate, less postoperative complications, and reduced hospital stay, as well as a higher R0 resection rate to avoid a positive surgical margin [12,13,14,15].
Despite all these advantages, ICG fluorescence navigation has some inherent drawbacks. First, ICG is usually injected intravenously a few days prior to the operation and bile metabolism malfunctions in diseased (especially cirrhotic) liver, and residual ICG will affect the observation [13, 16]. Second, regenerated nodules might show as false-positive if the bile duct is compressed [9]. Third, ICG fluorescence can only penetrate about 5–10 mm of tissue, so deep tumors cannot be detected superficially and positive- and negative-staining must be combined. More studies are needed to demonstrate the importance of ICG fluorescence navigation in laparoscopic hepatectomy.
We conducted this retrospective study, based on our 120-patient, single-center experience of laparoscopic hepatectomy, to investigate the role of ICG fluorescence navigation in laparoscopic hepatectomy and to assess the influence of the timing of ICG administration on intraoperative observation.
Methods
Patients and grou**
All patients who underwent laparoscopic hepatectomy between January, 2018 and December, 2019, were included in this retrospective study, with the exclusion of those under18 years of age, those with a history of prior upper abdominal surgery, and those who required conversion to open surgery. All operations were performed by the same team of surgeons who had completed the necessary training. The120 patients enrolled were divided into an ICG-FN group and a non-ICG-FN group, according to the availability of the ICG-FN imaging system. The ICG-FN group was divided further into a 0–3 days subgroup and a 4–7 days subgroup according to number of days preoperatively that ICG was administered. This retrospective study was approved by the Institutional Review Board of the First Affiliated Hospital, Nan**g Medical University (No. 2020-SR-124).
Surgical procedure and ICG fluorescence navigation
To obtain R0 resection and preserve maximal liver parenchyma, non-anatomic or anatomic resection was selected flexibly, depending on lesion-related characteristics and the preoperative liver function of the patient. There was no difference in the surgical procedure between the groups. The PINPOINT PC9000 (NOVADAQ, Canada) endoscopic system was used for fluorescence navigation. ICG was injected intravenously (0.5 mg/kg body weight) into a peripheral vein, 0–7 days before or during the operation, for tumor-specific or negative staining, respectively.
Observation indexes
The demographic characteristics (gender and age), lesion-related characteristics (lesion pathology type, number of involved lobes, and diameter of measurable lesion), and liver function parameters [degree of cirrhosis, volume of ascites, and ICG R15 (ICG retention rate after 15 min)] were obtained as baseline data in all patients. Observation indexes included the operative method (anatomical/non-anatomical resection), operative time, hilar occlusion time, intraoperative blood loss, intraoperative transfusion, postoperative complications, and postoperative hospital stay for all patients, as well as pathological evaluation of the surgical margin for malignancy in both groups. In the ICG-FN group, intraoperative imaging was assessed by both the surgeon and the assistant, who checked for clear staining in the target area, with visible boundaries and no residual ICG or diffusion in the non-target area.
Statistical analysis
Statistical analysis was performed using SPSS 22.0 software. The mean and standard deviation (SD) were calculated for normally distributed variables, with median and the 1st/3rd quartiles for skewed data. The Pearson χ2 test was used to compare differences in frequencies. A continuous corrected χ2 test was used if the theoretical value was between 1 and 5, and the Fisher-exact test was used if it was less than 1. Student's t test and Mann–Whitney U test were used to compare mean and median values between the groups. Spearman's rank correlation analysis was used to analyze the correlation between treatment measures and clinical observation indexes. Backward stepwise logistic regression models were used to assess the association between variables and clinical observations indexes (continuous variables were converted to categorical variables), after adjustment for ICG fluorescence navigation, gender, age, number of involved lobes, diameter of measurable lesion, degree of cirrhosis, volume of ascites, ICG R15, and operation method. A value of p < 0.05 was considered significant.
Results
Baseline characteristics in the ICG-FN and non-ICG-FN groups
There were 57 patients in the ICG-FN group and 63 patients in the non-ICG-FN group. There were no significant differences in the demographic characteristics (gender and age), lesion-related characteristics (pathology type, number of involved lobes, and diameter of measurable lesion), and liver function parameters (degree of cirrhosis, volume of ascites, and ICG R15) between the ICG-FN and non-ICG-FN groups (p > 0.05 for all; Table 1).
Operation and recovery indexes in the ICG-FN and non-ICG-FN groups
The operative time and intraoperative blood loss were significantly lower in the ICG-FN group than in the non ICG-FN group, being 160 (115–195) min vs. 180 (125–225) min (p = 0.035) and 100 (35–200) ml vs. 200 (100–400) ml (p = 0.025), respectively. The operation method (anatomical/non-anatomical resection), hilar occlusion time, intraoperative transfusion, incidence of postoperative complications, and postoperative hospital stay did not differ significantly between the groups (Table 2).
Pathological evaluation of the surgical margin for malignant tumors
Overall, there were 39 patients who underwent surgery for malignant disease, accounting for 68.4% and 61.9% of the total population in each group, with no difference in composition ratio (Table 1). Pathological evaluation of the surgical margin (Table 3) suggested that the R0 resection rate was comparable in the ICG-FN and non-ICG-FN groups (100.0 vs. 94.9%, p = 0.474). Therefore, we further compared the cases of a wide surgical margin (margin width > 10 mm) in both groups and found the rate of a wide surgical margin to be significantly higher in the ICG-FN group than in the non-ICG-FN group (92.3 vs. 74.4%, p = 0.033).
Intraoperative fluorescence imaging satisfaction analysis
Fluorescence imaging satisfaction was evaluated intraoperatively in the ICG-FN group by both the surgeon and the assistant. The imaging quality was not clear enough to proceed with surgery in 3 (7.5%) of the 40 patients in the 0–3 preoperative days subgroup, but no significance was indicated (p = 0.547; Table 4). The operative time, hilar occlusion time, intraoperative blood loss, and postoperative hospital stay were all comparable (p > 0.05 for all).
Spearman's rank correlation and backward stepwise logistic regression analysis of operative time and intraoperative blood loss for all patients
Spearman's rank correlation analysis revealed that the operative time (r = − 0.193, p = 0.035) and intraoperative blood loss (r = − 0.205, p = 0.025) were negatively correlated with ICG fluorescence navigation (Table 5), but not with gender, age, lesion pathology type, number of involved lobes, diameter of measurable lesion, degree of cirrhosis, volume of ascites, ICG R15, and hepatectomy method. Backward stepwise logistic regression analysis further indicated that the risk factor for operative time was the diameter of a measurable lesion (OR 1.221, 95% CI 1.052–1.419, p = 0.009), the risk factors for intraoperative blood loss were the diameter of a measurable lesion (OR 1.183, 95% CI 1.021–1.370, p = 0.025) and age (OR 1.035, 95% CI 1.003–1.069, p = 0.032), and that ICG fluorescence navigation was a protective factor (OR 0.446, 95% CI 0.205–0.967, p = 0.041) (Table 6).
Spearman's rank correlation and backward stepwise logistic regression analysis of wide surgical margin for malignant tumors
Spearman's rank correlation analysis suggested a positive correlation between a wide surgical margin and ICG fluorescence navigation (r = 0.241, p = 0.034; Table 7), but not to gender, age, lesion pathology type, number of involved lobes, diameter of measurable lesion, degree of cirrhosis, volume of ascites, ICG R15, or hepatectomy method. Backward stepwise logistic regression analysis illustrated that ICG fluorescence navigation was the only protective factor (OR 4.138, 95% CI 1.041–16.444, p = 0.044; Table 7) to obtain a wide surgical margin.
Effects of liver resection type on operation and recovery of all patients and the surgical margin for malignant tumors
There were no significant differences in operative time, hilar occlusion time, intraoperative blood loss, postoperative hospital stay, and wide or negative surgical margin rate between patients who underwent anatomical vs. those who underwent non-anatomical liver resection (Tables S1 and S2).
Discussion
Recent studies have demonstrated the safety and efficiency of ICG fluorescence navigation in laparoscopic hepatectomy [12,13,14,15]. This study also showed that the intraoperative and postoperative indexes of the ICG-FN group were comparable to or even better than those of the non-ICG-FN group. For patients with malignant tumors, the residual ICG inside the lesion may emit fluorescence under near-infrared light illumination, which helps the surgeon find the lesion quickly, especially if the tumor is in an “inconvenient” location such as the right posterior lobe [10, 11, 17]. The implementation of a negative staining technique is also helpful to identify the liver segment/lobe boundary and the resection plane. Energy devices such as the ultracision harmonic scalpel (Johnson & Johnson) are capable of breaking the liver parenchyma in the non-vascular area between the liver segments, reducing the need for vascular clips, reducing the operative time, preventing large vessel injury in the liver segments, and minimizing intraoperative blood loss. This was confirmed in our study. As a result of advances in surgical techniques, the overall intraoperative transfusion rate was low, with no difference between the groups. Although studies have shown that fluorescence navigation can reduce the incidence of bile leakage and liver abscess after hepatic segmentectomy and lobectomy [12], and minimize the postoperative hospital stay [13, 14], this study did not find that ICG fluorescence navigation reduced postoperative complications or the postoperative hospital stay. However, Spearman’s rank correlation and stepwise Logistic regression analysis indicated that ICG fluorescence navigation was associated with shorter operative time and less intraoperative blood loss. It was also a protective factor for less intraoperative blood loss, proving its safety and effectiveness. In this study, the liver resection type (anatomical vs. non-anatomical) did not affect operation time, hilar occlusion time, intraoperative blood loss, or postoperative hospital stay.
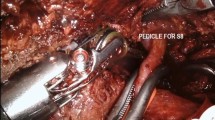
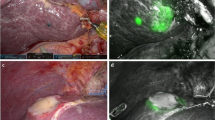
ICG dissolved in sterilized water was usually administrated preoperatively via a peripheral vein. ICG distributed in liver tissues with blood flow will be excreted through bile by normal hepatocytes, leading to tumor-specific staining, which enables the tumor to be visualized easily. As fluorescence penetrates the tissue to a depth of only 5–10 mm [6], superficial tumors near the liver capsular can be detected (Fig. 1a). To retain maximal liver parenchyma and reduce the postoperative complications caused by lack of liver volume, parenchymal-sparing non-anatomic resection could be performed, maintaining integrity around the tumors in the liver parenchyma and achieving a curative effect [18]. Intraoperative real-time navigated resection along the fluorescence boundaries was well accepted (Fig. 1b). Anatomic hepatectomy is a better option for non-neoplastic lesions such as hepatolithiasis and tumors located deep in the liver parenchyma not able to be well observed by tumor specific staining. Intraoperatively, the Glissonean pedicle of the lesion-involved segment/lobe was dissected and clipped (Fig. 2a, b), and then ICG was injected intravenously. ICG cannot be delivered into the area to be removed and the remnant liver was stained negatively (Fig. 2c). In the process of liver parenchyma resection, real-time fluorescence navigation was also used to obtain a satisfactory resection section (Fig. 2d). The combination of positive and negative staining can improve the success rate of ICG fluorescence navigation, although extensive clinical training and practical experience is required.
ICG was injected into segment 2 portal branch (black arrow) a. Segment 2 was stained immediately and a superficial tumor near the liver capsular was visualized on the fluorescence fusion image under illumination of near infrared light b. This segment was resected using ICG fluorescence navigation and left hepatic vein was indicated (white arrow) c
Left hepatic artery (a) and portal vein branch (b) were dissected and clipped. Then, ICG solution was injected peripheral intravenously. Boundary of hemiliver was apparent on the fluorescence fusion image while the right half stained but the left not (c). Exsection plane was marked and left semi-hepatectomy was accomplished (d)
Previous studies have suggested that the timing of preoperative ICG injection may affect the fluorescence imaging of tumor-specific staining. It is thought that the injection should not be given fewer than 4 days preoperatively; and that administration 0–3 days preoperatively may decrease the detection rate of tumors [19, 20]. However, the flexibility of medical resource allocation might be poorer and the waiting time before surgery would be prolonged in this setting. A small retrospective study indicated that a shorter injection time can also result in a satisfactory imaging effect [16]. However, as the ICG administration timing remains inconclusive, another cohort study was begun last year [21]. In our experience, administering ICG 0–3 days preoperatively results in comparable staining and imaging satisfaction to 4–7 days preoperatively (p = 0.547). Moreover, there were no significant differences in objective indexes, such as operative time, hilar occlusion time, intraoperative blood loss and postoperative hospital stay (p > 0.05 for all), when ICG was administered 0–3 days preoperatively. ICG injection timing closer to the operation day meant shorter preoperative waiting time and better medical resources allocation.
The surgical margin around a malignant tumor is another concern of hepatobiliary surgeons, and related to tumor recurrence and patient survival. R0 resection means that the surgical margin is at least 1 mm away from the tumor boundary and no tumor cells are visible under the microscope. R0 resection was reported to be associated with better long-term survival [22]. ICG fluorescence navigation facilitates the comprehensive application of positive and negative staining and real-time navigation technology to achieve R0 resection [15]. Furthermore, it was demonstrated that narrow resection margin (width ≤ 10 mm) was a predictor for overall survival [23]. A meta-analysis of 34 studies involving 11,147 hepatectomy patients concluded that patients with a wide margin (> 10 mm) had a better prognosis, suggesting that this should be one of the goals of hepatectomy [24]. Recent studies also identified that a wide surgical margin is predictive of a better long-term prognosis [25,26,27]. During laparoscopic surgery, the lack of tactile perception of laparoscopic forceps and the 2-dimensional image on the monitor in most centers might affect surgeons’ evaluation of the resection range, which could be insufficient to preserve liver parenchyma, resulting in a narrow margin or a positive margin. Our study found no difference in R0 resection rates between the groups, regardless of whether ICG fluorescence navigation was used, but a wide margin was easier to attain in the ICG-FN group. Spearman’s rank correlation and stepwise logistic regression analysis suggested that ICG fluorescence navigation was an important correlation factor and predictor of a wide margin. As a wide surgical margin should be a target of resection of malignant liver tumors [24,25,26,27], these results indicated that the patients in the ICG-FN group might have a lower postoperative recurrence rate and longer postoperative survival, although this still needs to be verified by long-term follow-up data.
In conclusion, laparoscopic hepatectomy is now performed widely because of its minimal invasion and enhanced recovery, although it requires skill, training, and practice by surgeons. As an emerging technology, ICG fluorescence navigation is safe and efficient in laparoscopic hepatectomy. It also helps to achieve a wide surgical margin, which often results in a better prognosis. The findings of this study suggest that the administration of ICG closer to the operation, 0–3-days preoperatively, is acceptable.
References
Cheung TT, Han HS, She WH, Chen KH, Chow PKH, Yoong BK, et al. The Asia Pacific consensus statement on laparoscopic liver resection for hepatocellular carcinoma: a report from the 7th Asia-Pacific primary liver cancer expert meeting held in Hong Kong. Liver Cancer. 2018;7:28–39.
Inoue Y, Arita J, Sakamoto T, Ono Y, Takahashi M, Takahashi Y, et al. Anatomical liver resections guided by 3-dimensional parenchymal staining using fusion indocyanine green fluorescence imaging. Ann Surg. 2015;262:105–11.
Urade T, Sawa H, Iwatani Y, Abe T, Fu**aka R, Murata K, et al. Laparoscopic anatomical liver resection using indocyanine green fluorescence imaging. Asian J Surg. 2020;43:362–8.
Ito D, Ishizawa T, Hasegawa K. Laparoscopic positive staining of hepatic segments using ICG-fluorescence imaging. J Hepatobiliary Pancreat Sci. 2020;27:441–3.
Aoki T, Murakami M, Koizumi T, Matsuda K, Fujimori A, Kusano T, et al. Determination of the surgical margin in laparoscopic liver resections using infrared indocyanine green fluorescence. Langenbecks Arch Surg. 2018;403:671–80.
Landsman ML, Kwant G, Mook GA, Zijlstra WG. Light-absorbing properties, stability, and spectral stabilization of indocyanine green. J Appl Physiol. 1976;40:575–83.
Ishizawa T, Saiura A, Kokudo N. Clinical application of indocyanine green-fluorescence imaging during hepatectomy. Hepatobiliary SurgNutr. 2016;5:322–8.
Qi C, Zhang H, Chen Y, Su S, Wang X, Huang X, et al. Effectiveness and safety of indocyanine green fluorescence imaging-guided hepatectomy for liver tumors: a systematic review and first meta-analysis. PhotodiagnosisPhotodynTher. 2019;28:346–53.
Ishizawa T, Fukushima N, Shibahara J, Masuda K, Tamura S, Aoki T, et al. Real-time identification of liver cancers by using indocyanine green fluorescent imaging. Cancer. 2009;115:2491–504.
Lim C, Vibert E, Azoulay D, Salloum C, Ishizawa T, Yoshioka R, et al. Indocyanine green fluorescence imaging in the surgical management of liver cancers: current facts and future implications. J ViscSurg. 2014;151:117–24.
Marino MV, Podda M, Fernandez CC, Ruiz MG, Fleitas MG. The application of indocyanine green-fluorescence imaging during robotic-assisted liver resection for malignant tumors: a single-arm feasibility cohort study. HPB (Oxford). 2019;22:422–31.
Marino MV, Builes RS, Gomez RM. The application of indocyanine green (ICG) staining technique during robotic-assisted right hepatectomy: with video. J GastrointestSurg. 2019;23:2312–3.
Zhang P, Luo H, Zhu W, Yang J, Zeng N, Fan Y, et al. Real-time navigation for laparoscopic hepatectomy using image fusion of preoperative 3D surgical plan and intraoperative indocyanine green fluorescence imaging. SurgEndosc. 2019;34:3449–59.
He K, Hong X, Chi C, Cai C, Wang K, Li P, et al. A new method of near-infrared fluorescence image-guided hepatectomy for patients with hepatolithiasis: a randomized controlled trial. SurgEndosc. 2020;34:4975–82.
Nomi T, Hokuto D, Yoshikawa T, Matsuo Y, Sho M. A novel navigation for laparoscopic anatomic liver resection using indocyanine green fluorescence. Ann SurgOncol. 2018;25:3982.
Lim HJ, Chiow AKH, Lee LS, Tan SS, Goh BK, Koh YX, et al. Novel method of intraoperative liver tumour localisation with indocyanine green and near-infrared imaging. Singapore Med J. 2019. https://doi.org/10.11622/smedj.2019137.
He JM, Zhen ZP, Ye Q, Mo JQ, Chen GH, Peng JX. Laparoscopic anatomical segment VII resection for hepatocellular carcinoma using the Glissonian approach with indocyanine green dye fluorescence. J GastrointestSurg. 2020;24:1228–9.
Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, Felekouras E, Dillhoff M, Schmidt C, et al. Parenchymal-sparing versus anatomic liver resection for colorectal liver metastases: a systematic review. J GastrointestSurg. 2017;21:1076–85.
Lwin TM, Hoffman RM, Bouvet M. Regarding the applications of fusion-fluorescence imaging using indocyanine green in laparoscopic hepatectomy. TranslGastroenterolHepatol. 2017;2:70.
Herman P, Coelho FF, Perini MV, Lupinacci RM, D’Albuquerque LA, Cecconello I. Hepatocellular adenoma: an excellent indication for laparoscopic liver resection. HPB (Oxford). 2012;14:390–5.
Gon H, Komatsu S, Murakami S, Kido M, Tanaka M, Kuramitsu K, et al. Real-time navigation during hepatectomy using fusion indocyanine green-fluorescence imaging: protocol for a prospective cohort study. BMJ Open. 2019;9:e030233.
Lang H, Sotiropoulos GC, Brokalaki EI, Schmitz KJ, Bertona C, Meyer G, et al. Survival and recurrence rates after resection for hepatocellular carcinoma in noncirrhotic livers. J Am CollSurg. 2007;205:27–36.
Liu W, Sun Y, Zhang L, **ng BC. Negative surgical margin improved long-term survival of colorectal cancer liver metastases after hepatic resection: a systematic review and meta-analysis. Int J Colorectal Dis. 2015;30:1365–73.
Margonis GA, Sergentanis TN, Ntanasis-Stathopoulos I, Andreatos N, Tzanninis IG, Sasaki K, et al. Impact of surgical margin width on recurrence and overall survival following r0 hepatic resection of colorectal metastases: a systematic review and meta-analysis. Ann Surg. 2018;267:1047–55.
Shi C, Zhao Q, Liao B, Dong Z, Wang C, Yang J, et al. Anatomic resection and wide resection margin play an important role in hepatectomy for hepatocellular carcinoma with peritumoural micrometastasis. ANZ J Surg. 2019;89:E482–6.
Yang P, Si A, Yang J, Cheng Z, Wang K, Li J, et al. A wide-margin liver resection improves long-term outcomes for patients with HBV-related hepatocellular carcinoma with microvascular invasion. Surgery. 2019;165:721–30.
Watanabe Y, Matsuyama Y, Izumi N, Kubo S, Kokudo N, Sakamoto M, et al. Effect of surgical margin width after R0 resection for intrahepatic cholangiocarcinoma: a nationwide survey of the Liver Cancer Study Group of Japan. Surgery. 2020;167:793–802.
Funding
None.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
We have no conflicts of interest to declare.
Ethical statement
The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. This study was approved by the Institutional Review Board of the First Affiliated Hospital, Nan**g Medical University (No. 2020-SR-124).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lu, H., Gu, J., Qian, Xf. et al. Indocyanine green fluorescence navigation in laparoscopic hepatectomy: a retrospective single-center study of 120 cases. Surg Today 51, 695–702 (2021). https://doi.org/10.1007/s00595-020-02163-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00595-020-02163-8