Abstract
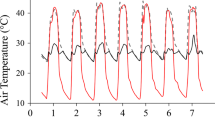
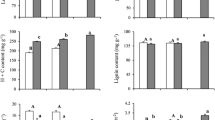
The aim of this study was to assess the temperature response of photosynthesis in rubber trees (Hevea brasiliensis Müll. Arg.) to provide data for process-based growth modeling, and to test whether photosynthetic capacity and temperature response of photosynthesis acclimates to changes in ambient temperature. Net CO2 assimilation rate (A) was measured in rubber saplings grown in a nursery or in growth chambers at 18 and 28°C. The temperature response of A was measured from 9 to 45°C and the data were fitted to an empirical model. Photosynthetic capacity (maximal carboxylation rate, V cmax, and maximal light driven electron flux, J max) of plants acclimated to 18 and 28°C were estimated by fitting a biochemical photosynthesis model to the CO2 response curves (A–C i curves) at six temperatures: 15, 22, 28, 32, 36 and 40°C. The optimal temperature for A (T opt) was much lower in plants grown at 18°C compared to 28°C and nursery. Net CO2 assimilation rate at optimal temperature (A opt), V cmax and J max at a reference temperature of 25°C (V cmax25 and J max25) as well as activation energy of V cmax and J max (E aV and E aJ) decreased in individuals acclimated to 18°C. The optimal temperature for V cmax and J max could not be clearly defined from our response curves, as they always were above 36°C and not far from 40°C. The ratio J max25/V cmax25 was larger in plants acclimated to 18°C. Less nitrogen was present and photosynthetic nitrogen use efficiency (V cmax25/N a) was smaller in leaves acclimated to 18°C. These results indicate that rubber saplings acclimated their photosynthetic characteristics in response to growth temperature, and that higher temperatures resulted in an enhanced photosynthetic capacity in the leaves, as well as larger activation energy for photosynthesis.



Similar content being viewed by others
References
Alam B, Jacob J (2002) Overproduction of photosynthetic electrons is associated with chilling injury in green leaves. Photosynthetica 40:91–95. doi:10.1023/A:1020110710726
Alam B, Nair DB, Jacob J (2005) Low temperature stress modifies the photochemical efficiency of a tropical tree species Hevea brasiliensis: effects of varying concentration of CO2 and photon flux density. Photosynthetica 43:247–252. doi:10.1007/s11099-005-0040-z
Atkin OK, Loveys BR, Atkinson LJ, Pons TL (2006a) Phenotypic plasticity and growth-temperature: understanding inter-specific variability. J Exp Bot 57:267–281. doi:10.1093/jxb/erj029
Atkin OK, Scheurwater I, Pons TL (2006b) High thermal acclimation potential of both photosynthesis and respiration in two lowland Plantago species in contrast to an alpine congeneric. Glob Change Biol 12:500–515. doi:10.1111/j.1365-2486.2006.01114.x
Bernacchi CJ, Singsaas EL, Pimentel C, Portis AR Jr, Long SP (2001) Improved temperature response functions for models of Rubisco-limited photosynthesis. Plant Cell Environ 24:253–259. doi:10.1111/j.1365-3040.2001.00668.x
Berry J, Björkman O (1980) Photosynthetic response and adaptation to temperature in higher plants. Annu Rev Plant Physiol 31:491–543. doi:10.1146/annurev.pp.31.060180.002423
Bertin N, Tchamitchian M, Baldet P, Devaux C, Brunel B, Gary C (1999) Contribution of carbohydrate pools to the variations in leaf mass per area within a tomato plant. New Phytol 143:53–61. doi:10.1046/j.1469-8137.1999.00436.x
Bunce JA (2000) Acclimation of photosynthesis to temperature in eight cool and warm climate herbaceous C3 species: temperature dependence of parameters of a biochemical photosynthesis model. Photosynth Res 63:59–67. doi:10.1023/A:1006325724086
von Caemmerer S (2000) Biochemical models of leaf photosynthesis. CSIRO Publishing, Canberra
Dreyer E, Le Roux X, Montpied P, Daudet FA, Masson F (2001) Temperature response of leaf photosynthetic capacity in seedlings from seven temperature tree species. Tree Physiol 21:223–232
Ethier GJ, Livingston NJ (2004) On the need to incorporate sensitivity to CO2 transfer conductance into the Farquhar-von Caemmerer-Berry leaf photosynthesis model. Plant Cell Environ 27:137–153. doi:10.1111/j.1365-3040.2004.01140.x
Farquhar GD, von Caemmerer S (1982) Modelling of photosynthetic response to environmental conditions. In: Lange OL, Nobel PL, Osmon CB, Ziegler H (eds) Encyclopedia of plant physiology, NS. Physiological plant ecology II, vol 12 B. Springer, Berlin, pp 550–587
Farquhar GD, von Caemmerer S, Berry JA (1980) A biochemical model of photosynthetic CO2 assimilation in leaves of C3 species. Planta 149:78–90. doi:10.1007/BF00386231
Ferrar PJ, Slatyer RO, Vranjic JA (1989) Photosynthetic temperature acclimation in Eucalyptus species from diverse habitats, and a comparison with Nerium oleander. Aust J Plant Physiol 16:199–217
Ghouil H, Montpied P, Epron D, Ksontini M, Hanchi B, Dreyer E (2003) Thermal optima of photosynthetic functions and thermostability of photochemistry in cork oak seedlings. Tree Physiol 23:1031–1039
Harley PC, Tenhunen JD (1991) Modeling the photosynthetic response of C3 leaves to environmental factors. In: Modeling crop photosynthesis-from biochemistry to canopy, vol 19. American Society of Agronomy and Crop Science Society of America, Madison, pp 17–39
Harley PC, Thomas RB, Reynolds JF, Strain BR (1992) Modelling photosynthesis of cotton grown in elevated CO2. Plant Cell Environ 15:271–282. doi:10.1111/j.1365-3040.1992.tb00974.x
Hikosaka K, Hanba YT, Hirose T, Terashima I (1998) Photosynthetic nitrogen-use efficiency in woody and herbaceous plants. Funct Ecol 12:896–905. doi:10.1046/j.1365-2435.1998.00272.x
Hikosaka K, Murakami A, Hirose T (1999) Balancing carboxylation and regeneration of ribulose-1,5-bisphosphate in leaf photosynthesis: temperature acclimation of an evergreen tree, Quercus myrsinaefolia. Plant Cell Environ 22:841–849. doi:10.1046/j.1365-3040.1999.00442.x
Hikosaka K, Ishikawa K, Borjigidai A, Muller O, Onoda Y (2006) Temperature acclimation of photosynthesis: mechanisms involved in the changes in temperature dependence of photosynthetic rate. J Exp Bot 57:291–302. doi:10.1093/jxb/erj049
June T, Evans JR, Farquhar GD (2004) A simple new equation for the reversible temperature dependence of photosynthetic electron transport: a study on soybean leaf. Funct Plant Biol 31:275–283. doi:10.1071/FP03250
Kattge J, Knorr W (2007) Temperature acclimation in a biochemical model of photosynthesis: a reanalysis of data from 36 species. Plant Cell Environ 30:1176–1190. doi:10.1111/j.1365-3040.2007.01690.x
Le Roux X, Grand S, Dreyer E, Duadet FA (1999) Parameterization and testing of a biochemically based photosynthesis model for walnut (Juglans regia) trees and seedlings. Tree Physiol 19:181–188
Leuning R (1997) Scaling to a common temperature improves the correlation between the photosynthesis parameters J max and V cmax. J Exp Bot 48:345–347. doi:10.1093/jxb/48.2.345
Makino A, Nakano H, Mae T (1994) Effects of growth temperature on the response of ribulose-1,5-bisphosphate carboxylase, electron transport components, and sucrose synthesis enzymes to leaf nitrogen in rice, and their relationships to photosynthesis. Plant Physiol 105:1231–1238
Mason N, Hughes P, McMullan R, Houghton JT (2001) Vegetation growth and the carbon balance. In: Introduction to environmental physics: planet Earth, life and climate. Taylor & Francis group, London, pp 363–396
Mediavilla S, Escudero A, Heilmeier H (2001) Internal leaf anatomy and photosynthetic resource-use efficiency: interspecific and intraspecific comparisons. Tree Physiol 21:251–259
Medlyn BE, Dreyer E, Ellsworth D, Forstreuter M, Harley PC, Kirschbaum MUF et al (2002a) Temperature response of parameters of a biochemically based model of photosynthesis. II. A review of experimental data. Plant Cell Environ 25:1167–1179. doi:10.1046/j.1365-3040.2002.00891.x
Medlyn BE, Loustau D, Delzon S (2002b) Temperature response of parameters of a biochemically based model of photosynthesis. I. Seasonal changes in mature maritime pine (Pinus pinaster Ait.). Plant Cell Environ 25:1155–1165. doi:10.1046/j.1365-3040.2002.00890.x
Niinemets Ü, Tenhunen JD (1997) A model separating leaf structural and physiological effects on carbon gain along light gradients for the shade tolerant species Acer saccharum. Plant Cell Environ 20:845–856. doi:10.1046/j.1365-3040.1997.d01-133.x
Niinemets Ü, Oja V, Kull O (1999a) Shape of leaf photosynthetic electron transport versus temperature response curve is not constant along canopy light gradients in temperate deciduous trees. Plant Cell Environ 22:1497–1513. doi:10.1046/j.1365-3040.1999.00510.x
Niinemets Ü, Tenhunen JD, Canta NR, Chaves M, Faria T, Pereira JS et al (1999b) Interactive effects of nitrogen and phosphorus on the acclimation potential of foliage photosynthetic properties of cork oak, Quercus suber, to elevated CO2 concentrations. Glob Change Biol 5:455–470. doi:10.1046/j.1365-2486.1999.00241.x
Onoda Y, Hikosaka K, Hirose T (2005a) Seasonal change in the balance between capacities of RuBP carboxylation and RuBP regeneration affects CO2 response of photosynthesis in Polygonum cuspidatum. J Exp Bot 56:755–763. doi:10.1093/jxb/eri052
Onoda Y, Hikosaka K, Hirose T (2005b) The balance between RuBP carboxylation and RuBP regeneration: a mechanism underlying the interspecific variation in acclimation of photosynthesis to seasonal change in temperature. Funct Plant Biol 32:903–910. doi:10.1071/FP05024
Raj S, Das G, Pothen J, Dey SK (2005) Relationship between latex yield of Hevea brasiliensis and antecedent environmental parameters. Int J Biometeorol 49:189–196. doi:10.1007/s00484-004-0222-6
Rao PS, Saraswathyamma CK, Sethuraj MR (1998) Studies on the relationship between yield and meteorological parameters of para rubber tree (Hevea brasiliensis). Agric For Meteorol 90:235–245. doi:10.1016/S0168-1923(98)00051-3
Robakowski P, Monpied P, Dreyer E (2002) Temperature response of photosynthesis of silver fir (Abies alba Mill.) seedlings. Ann For Sci 59:163–170. doi:10.1051/forest:2002003
Sage RF, Kubien DS (2007) The temperature response of C3 and C4 photosynthesis. Plant Cell Environ 30:1086–1106. doi:10.1111/j.1365-3040.2007.01682.x
Salvucci ME, Crafts-Brandner SJ (2004) Relationship between the heat tolerance of photosynthesis and the thermal stability of Rubisco activase in plants from contrasting thermal environments. Plant Physiol 134:1460–1470. doi:10.1104/pp.103.038323
Slatyer RO (1977a) Altitudinal variation in photosynthetic characteristics of snow gum, Eucalyptus pauciflora Sieb. ex Spreng. III. Temperature response of material grown in contrasting thermal environments. Aust J Plant Physiol 4:301–312
Slatyer RO (1977b) Altitudinal variation in the photosynthetic characteristics of snow gum, Eucalyptus pauciflora Sieb. Ex Spreng. IV. Temperature response of four populations grown at different temperatures. Aust J Plant Physiol 4:583–594
Slatyer RO, Morrow PA (1977) Altitudinal variation in photosynthetic characteristics of snow gum, Eucalyptus pauciflora Sieb. ex Spreng. I. Seasonal changes under field conditions in the snowy mountains area of south-eastern Australia. Aust J Plant Physiol 25:1–20
Takashima T, Hikosaka K, Hirose T (2004) Photosynthesis or persistence: nitrogen allocation in leaves of evergreen and deciduous Quercus species. Plant Cell Environ 27:1047–1054. doi:10.1111/j.1365-3040.2004.01209.x
Usami T, Lee J, Oikawa T (2001) Interactive effects of increased temperature and CO2 on the growth of Quercus myrsinaefolia saplings. Plant Cell Environ 24:1007–1019. doi:10.1046/j.1365-3040.2001.00753.x
Walcroft AS, Kelliher FM (1997) The response of photosynthetic model parameters to temperature and nitrogen concentration in Pinus radiata D. Don. Plant Cell Environ 20:1338–1348. doi:10.1046/j.1365-3040.1997.d01-31.x
Warren CR (2008) Does growth temperature affect the temperature responses of photosynthesis and internal conductance to CO2? A test with Eucalyptus regnans. Tree Physiol 28:11–19
Warren CR, Dreyer E (2006) Temperature response of photosynthesis and internal conductance to CO2: results from two independent approaches. J Exp Bot 57:3057–3067. doi:10.1093/jxb/erl067
Wullschleger SD (1993) Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/C i curves from 109 species. J Exp Bot 44:907–920. doi:10.1093/jxb/44.5.907
Yamasaki T, Yamakawa T, Yamane Y, Koike H, Satoh K, Katoh S (2002) Temperature acclimation of photosynthesis and related changes in photosystem II electron transport in winter wheat. Plant Physiol 128:1087–1097. doi:10.1104/pp.010919
Yamori W, Nokuchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28:536–547. doi:10.1111/j.1365-3040.2004.01299.x
Yamori W, Suzuki K, Noguchi K, Nakai M, Terashima I (2006) Effects of rubisco kinetics and rubisco activation state on the temperature dependence of the photosynthetic rate in spinach leaves from contrasting growth temperatures. Plant Cell Environ 29:1659–1670. doi:10.1111/j.1365-3040.2006.01550.x
Acknowledgments
The authors are grateful to Sornprach Thanisawanyangkura, Kumut Sangkhasila, Krissada Sangsing, Ela Frak and Kathy Steppe for helpful advice, to Jate Sathornkich, Pongpan Siripornpakdeekul and Rungroj Jitthongchai for help in construction of the air-temperature control system, to Marc Vandame for generous help during the experiment and help in leaf sample analysis, to Stéphane Ploquin for taking care of plants, to Michael Smith for helpful suggestion and providing some external parts of CO2 injector of LI-6400 in France, to the Michelin company in Clermont-Ferrand for providing rubber saplings and to **g Mai for help in growing plants and taking care of the saplings. This study was supported by DORAS Rubber Project-Kasetsart University, The Franco–Thai Cooperation Program in Higher Education and Research 2005, Cirad Incentive Program ‘Support to PhDs’, CRN (Cooperative Research Network) Scholarship-Thailand and Chulalongkorn University.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Hampp.
Rights and permissions
About this article
Cite this article
Kositsup, B., Montpied, P., Kasemsap, P. et al. Photosynthetic capacity and temperature responses of photosynthesis of rubber trees (Hevea brasiliensis Müll. Arg.) acclimate to changes in ambient temperatures. Trees 23, 357–365 (2009). https://doi.org/10.1007/s00468-008-0284-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00468-008-0284-x