Abstract
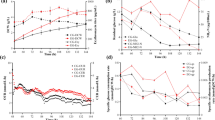
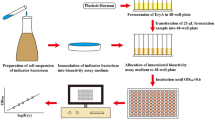
In the current study, the effect of different available nitrogen sources on erythromycin fermentation by Saccharopolyspora erythraea No. 8 is evaluated. Three different combinations of corn steep liquor and yeast powder were developed to investigate their impacts on erythromycin production. The results indicate that the optimal combination of available nitrogen sources was 10.0 g/L corn steep liquor and 4.0 g/L yeast power, generating a maximum yield of erythromycin of 13672 U/mL. To explore the effects of nitrogen perturbations on cell metabolism, metabolic flux analyses were performed and compared under different conditions. A high flux pentose phosphate pathway provided more NADPH for erythromycin synthesis via nitrogen optimization. Moreover, high n-propanol specific consumption rate enhanced erythromycin synthesis and n-propanol flowed into the central carbon metabolism by methylmalonyl-CoA node. These results indicate that the selection of an appropriate organic nitrogen source is essential for cell metabolism and erythromycin synthesis, and this is the first report of the successful application of available nitrogen source combinations in industrial erythromycin production.



Similar content being viewed by others
References
Wiley PF, Gerzon K, Flynn EH, Weaver O, Quarck UC, Chauvette RR, Monahan R (1957) Erythromycin. X1. Structure of erythromycin. J Am Chem Soc 79(22):6062–6070
Mironov VA, Sergienko OV, Nastasyak IN, Danilenko VN (2004) Biogenesis and Regulation of Biosynthesis of Erythromycins in Saccharopolyspora erythraea. Appl Biochem Microbiol 40(6):531–541
Taubman SB, Young FE, Corcoran JW (1963) Antibiotic glycosides, IV. studies on the mechanism of erythromycin resistance in Bacillus subtilis. Proc Natl Acad Sci USA 50(5):955–962
Taubman SB, Jones NR, Young FE, Corcoran JW (1966) Sensitivity and resistance to erythromycin in Bacillus subtilis 168: the ribosomal binding of erythromycin and chloramphenicol. Biochim Biophys Acta 123(2):438–440
Baltz RH (2006) Molecular engineering approaches to peptide, polyketide and other antibiotics. Nat Biotechnol 24(12):1533–1540
Chen CC, Hong M, Chu J, Huang MZ, Ouyang LM, Tian XW, Zhuang YP (2017) Blocking the flow of propionate into TCA cycle through a mutB knockout leads to a significant increase of erythromycin production by an industrial strain of Saccharopolyspora erythraea. Bioprocess Biosyst Eng 40(2):201–209
Wang Y, Wang YG, Chu J, Zhuang YP, Zhang LX, Zhang SL (2007) Improved production of erythromycin A by expression of a heterologous gene encoding S-adenosylmethionine synthetase. Appl Microbiol Biotechnol 75(4):837–842
Minas W, Brunker P, Kallio PT, Bailey JE (1998) Improved erythromycin production in a genetically engineered industrial strain of Saccharopolyspora erythraea. Biotechnol Prog 14(4):561–566
El-Enshasy HA, Mohamed NA, Farid MA, El-Diwany AI (2008) Improvement of erythromycin production by Saccharopolyspora erythraea in molasses based medium through cultivation medium optimization. Bioresour Technol 99(10):4263–4268
Devi CS, Saini A, Rastogi S, Naine SJ, Mohanasrinivasan V (2015) Strain improvement and optimization studies for enhanced production of erythromycin in bagasse based medium using Saccharopolyspora erythraea MTCC 1103. 3 Biotech 5(1):23–31
Zou X, Hang HF, Chen CF, Chu J, Zhuang YP, Zhang SL (2008) Application of oxygen uptake rate and response surface methodology for erythromycin production by Saccharopolyspora erythraea. J Ind Microbiol Biotechnol 35(12):1637–1642
Potvin J, Péringer P (1994) Ammonium regulation in Saccharopolyspora erythraea. Part I: Growth and antibiotic production. Biotechnol Lett 16(1):63–68
Martin JF, Demain AL (1980) Control of antibiotic biosynthesis. Microbiol Rev 44(2):230–251
Rafieenia R (2013) Effect of nutrients and culture conditions on antibiotic synthesis in Streptomycetes. Asian J Pharm Hea Sci 3(3):810–815
Zou X, Hang HF, Chu J, Zhuang YP, Zhang SL (2009) Enhancement of erythromycin A production with feeding available nitrogen sources in erythromycin biosynthesis phase. Bioresour Technol 100(13):3358–3365
Lara AR, Galindo E, Ramírez OT, Palomares LA (2006) Living with heterogeneities in bioreactors. Mol Biotechnol 34(3):355–381
Chen CF, Qi XC, Qian JC, Zhuang YP, Chu J, Zhang SL (2009) Effects of the dissolved oxygen on the erythromycin components of recombinant strain Saccharopolyspora erythraea ZLl004 fermentation. Chin J Antibio 34(11):659–663
Chen Y, Wang ZJ, Chu J, Zhuang YP, Zhang SL, Yu XG (2013) Significant decrease of broth viscosity and glucose consumption in erythromycin fermentation by dynamic regulation of ammonium sulfate and phosphate. Bioresour Technol 134(2):173–179
Chen Y, Huang MZ, Wang ZJ, Chu J, Zhuang YP, Zhang SL (2013) Controlling the feed rate of glucose and propanol for the enhancement of erythromycin production and exploration of propanol metabolism fate by quantitative metabolic flux analysis. Bioprocess Biosyst Eng 36(10):1445–1453
Zou X, Hang HF, Chu J, Zhuang YP, Zhang SL (2009) Oxygen uptake rate optimization with nitrogen regulation for erythromycin production and scale-up from 50 L to 372 m3 scale. Bioresour Technol 100(3):1406–1412
Zou X, Zeng W, Chen CF, Qi XC, Qian JC, Chu J, Zhuang YP, Zhang SL, Li WJ (2010) Fermentation optimization and industrialization of recombinant Saccharopolyspora erythraea strains for improved erythromycin a production. Biotechnol Bioprocess Eng 15(6):959–968
Zhao HT, Pang KY, Lin WL, Wang ZJ, Gao DQ, Guo MJ, Zhuang YP (2016) Optimization of the n-propanol concentration and feedback control strategy with electronic nose in erythromycin fermentation processes. Process Biochem 51(2):195–203
Maeda RN, Silva MMPD, Anna LMMS Jr (2010) Nitrogen source optimization for cellulase production by Penicillium funiculosum, using a sequential experimental design methodology and the desirability function. Appl Biochem Biotechnol 161(1–8):411–422
Corcoran JW (1981) Biochemical mechanisms in the biosynthesis of the erythromycins. Springer, Berlin
Reeves AR, Brikun IA, Cernota WH, Leach BI, Gonzalez MC, Weber JM (2007) Engineering of the methylmalonyl-CoA metabolite node for increased erythromycin production in oil-based fermentations of Saccharopolyspora erythraea. Metab Eng 9(3):293–303
Jung WS, Yoo YJ, Park JW, Park SR, Han AR, Ban YH, Kim EJ, Kim E, Yoon YJ (2011) A combined approach of classical mutagenesis and rational metabolic engineering improves rapamycin biosynthesis and provides insights into methylmalonyl-CoA precursor supply pathway in Streptomyces hygroscopicus ATCC 29253. Appl Microbiol Biotechnol 91(5):1389–1397
Li YY, Chang X, Yu WB, Li H, Ye ZQ, Yu H, Liu BH, Zhang Y, Zhang SL, Ye BC (2013) Systems perspectives on erythromycin biosynthesis by comparative genomic and transcriptomic analyses of S. erythraea E3 and NRRL23338 strains. BMC Genom 14(1):523
Reeves AR, Brikun IA, Cernota WH, Leach BI, Gonzalez MC, Weber JM (2006) Effects of methylmalonyl-CoA mutase gene knockouts on erythromycin production in carbohydrate-based and oil-based fermentations of Saccharopolyspora erythraea. J Ind Microbiol Biotechnol 33(7):600–609
Kumpfmüller J, Methling K, Fang L, Pfeifer BA, Lalk M, Schweder T (2016) Production of the polyketide 6-deoxyerythronolide B in the heterologous host Bacillus subtilis. Appl Microbiol Biotechnol 100(3):1209–1220
Karničar K, Drobnak I, Petek M, Magdevska V, Horvat J, Vidmar R, Baebler Š, Rotter A, Jamnik P, Fujs Š (2016) Integrated omics approaches provide strategies for rapid erythromycin yield increase in Saccharopolyspora erythraea. Microb Cell Fact 15(1):93
Lee WH, Chin YW, Han NS, Kim MD, Seo JH (2011) Enhanced production of GDP-L-fucose by overexpression of NADPH regenerator in recombinant Escherichia coli. Appl Microbiol Biotechnol 91(4):967–976
Ahmad I, Shim WY, Jeon WY, Yoon BH, Kim JH (2012) Enhancement of xylitol production in Candida tropicalis by co-expression of two genes involved in pentose phosphate pathway. Bioprocess Biosyst Eng 35(1–2):199–204
Potvin J, Péringer P (1993) Influence of N-propanol on growth and antibiotic production by an industrial strain of Streptomyces erythreus under different nutritional conditions. Biotechnol Lett 15(5):455–460
Guo Q, Chu J, Zhuang YP, Gao Y (2016) Controlling the feed rate of propanol to optimize erythromycin fermentation by on-line capacitance and oxygen uptake rate measurement. Bioprocess Biosyst Eng 39(2):255–265
Chen Y, Wang Z, Chu J, ** B, Zhuang Y (2015) The glucose RQ-feedback control leading to improved erythromycin production by a recombinant strain Saccharopolyspora erythraea ZL1004 and its scale-up to 372-m3 fermenter. Bioprocess Biosyst Eng 38(1):105–112
Chen Y, Deng W, Wu JQ, Qian JC, Chu J, Zhuang YP, Zhang SL, Liu W (2008) Genetic modulation of the overexpression of tailoring genes eryK and eryG leading to the improvement of erythromycin a purity and production in Saccharopolyspora erythraea fermentation. Appl Environ Microbiol 74(6):1820–1828
Yao LL, Liao CH, Huang G, Zhou Y, Rigali S, Zhang BC, Ye BC (2014) GlnR-mediated regulation of nitrogen metabolism in the actinomycete Saccharopolyspora erythraea. Appl Microbiol Biotechnol 98(18):7935–7948
Liao CH, Yao L, Xu Y, Liu WB, Zhou Y, Ye BC (2015) Nitrogen regulator GlnR controls uptake and utilization of non-phosphotransferase-system carbon sources in actinomycetes. Proc Natl Acad Sci USA 112(51):15630–15635
Acknowledgements
This work was financially supported by the National Key Research and Development Program (2017YFB0309302), the National Basic Research Program of China (973 Program) (No. 2012CB721006), National Natural Science Foundation of China (No. 21276081) and the Fundamental Research Funds for the Central Universities (22221818014).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Zhang, Q., Hang, H., Tian, X. et al. Combined available nitrogen resources enhanced erythromycin production and preliminary exploration of metabolic flux analysis under nitrogen perturbations. Bioprocess Biosyst Eng 42, 1747–1756 (2019). https://doi.org/10.1007/s00449-019-02171-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-019-02171-0