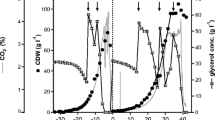
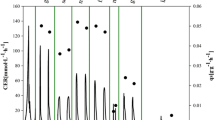
Abstract
Recombinant protein synthesis in Pichia pastoris is generally controlled by the strong methanol inducible AOX1 promoter which is repressed by glucose and glycerol. In shake flasks, commonly one or two methanol pulses are added per day for induction. Such pulse feeding procedure leads to carbon starvation phases, which may enhance proteolytic activities and, therefore, cause product losses. Starvation between the methanol pulses could be avoided with a continuous enzymatic feed of glucose from a glucose-based polymer. The amount of glucose was low enough to prevent AOX1 repression by glucose. Energy and carbon were continuously supplied for cell maintenance resulting in significantly increased cell densities and product activities, as shown here at the example of a fungal lipase expressed in P. pastoris. A threefold improvement in measured product activity was obtained by applying enzymatic glucose feed and a further improvement was achieved by applying a defined mixture of ammonium compounds. The strategy described here simplifies the general procedure in shaken cultures by allowing the direct continuation of the cultivation from glucose to the methanol-based production phase without a medium change. It is easily applicable to multiwell plates and thus beneficial for high throughput applications.



Similar content being viewed by others
References
Couderc R, Baratti J (1980) Oxidation of methanol by the yeast Pichia pastoris. Purification and properties of alcohol oxidase. Agric Biol Chem 44:2279–2289
Jahic M et al (2002) Modeling of growth and energy metabolism of Pichia pastoris producing a fusion protein. Bioprocess Biosyst Eng 24:385–393
Cereghino JL, Cregg JM (2000) Heterologous protein expression in the methylotrophic yeast Pichia pastoris. FEMS Microbiol Rev 24:45–66
Tschopp JF et al (1987) Expression of the lacZ gene from two methanol-regulated promoters in Pichia pastoris. Nucleic Acids Res 15:3859–3876
Boettner M et al (2002) High-throughput screening for expression of heterologous proteins in the yeast Pichia pastoris. J Biotechnol 99:51–62
Invitrogen (2010) Pichia expression kit for expression of recombinant proteins in Pichia pastoris. K1710-01
Ruottinen M et al (2008) Improved production of human type II procollagen in the yeast Pichia pastoris in shake flasks by a wireless-controlled fed-batch system. BMC Biotechnol 8:33
Katakura Y et al (1998) Effect of methanol concentration on the production of human [beta]2-glycoprotein I domain V by a recombinant Pichia pastoris: a simple system for the control of methanol concentration using a semiconductor gas sensor. J Ferment Bioeng 86:482–487
Zhang W et al (2000) Modeling Pichia pastoris growth on methanol and optimizing the production of a recombinant protein, the heavy-chain fragment C of botulinum neurotoxin, serotype A. Biotechnol Bioeng 70:1–8
Curvers S et al (2001) Human chymotrypsinogen B production with Pichia pastoris by integrated development of fermentation and downstream processing. Part 1. Fermentation. Biotechnol Prog 17:495–502
Jahic M et al (2003) Temperature limited fed-batch technique for control of proteolysis in Pichia pastoris bioreactor cultures. Microb Cell Fact 2:6
Zhu TC et al (2011) Understanding the effect of foreign gene dosage on the physiology of Pichia pastoris by transcriptional analysis of key genes. Appl Microbiol Biotechnol 89:1127–1135
Celik E, Calik P, Oliver SG (2010) Metabolic flux analysis for recombinant protein production by Pichia pastoris using dual carbon sources: effects of methanol feeding rate. Biotechnol Bioeng 105:317–329
Gao MJ et al (2012) Methanol/sorbitol co-feeding induction enhanced porcine interferon-alpha production by Pichia pastoris associated with energy metabolism shift. Bioprocess Biosyst Eng 35:1125–1136
Guerrero-Olazaran M et al (2009) Recombinant shrimp (Litopenaeus vannamei) trypsinogen production in Pichia pastoris. Biotechnol Prog 25:1310–1316
Inan M, Meagher MM (2001) Non-repressing carbon sources for alcohol oxidase (AOX1) promoter of Pichia pastoris. J Biosci Bioeng 92:585–589
Niu H et al (2013) A quantitative study of methanol/sorbitol co-feeding process of a Pichia pastoris Mut +/pAOX1-lacZ strain. Microb Cell Fact 12:33
Thorpe ED, d’Anjou MC, Daugulis AJ (1999) Sorbitol as a non-repressing carbon source for fed-batch fermentation of recombinant Pichia pastoris. Biotechnol Lett 21:669–672
Zhu T et al (2013) Transcriptional investigation of the effect of mixed feeding to identify the main cellular stresses on recombinant Pichia pastoris. J Ind Microbiol Biotechnol 40:183–189
Abad S et al (2010) Stepwise engineering of a Pichia pastoris D-amino acid oxidase whole cell catalyst. Microb Cell Fact 9:24
Holmes WJ et al (2009) Develo** a scalable model of recombinant protein yield from Pichia pastoris: the influence of culture conditions, biomass and induction regime. Microb Cell Fact 8:35
Jungo C, Marison I, von Stockar U (2007) Mixed feeds of glycerol and methanol can improve the performance of Pichia pastoris cultures: a quantitative study based on concentration gradients in transient continuous cultures. J Biotechnol 128:824–837
Bhambure R, Kumar K, Rathore AS (2011) High-throughput process development for biopharmaceutical drug substances. Trends Biotechnol 29:127–135
Panula-Perala J et al (2008) Enzyme controlled glucose auto-delivery for high cell density cultivations in microplates and shake flasks. Microb Cell Fact 7:31
Krause M et al (2010) A novel fed-batch based cultivation method provides high cell-density and improves yield of soluble recombinant proteins in shaken cultures. Microb Cell Fact 9:11
Minning S, Schmidt-Dannert C, Schmid RD (1998) Functional expression of Rhizopus oryzae lipase in Pichia pastoris: high-level production and some properties. J Biotechnol 66:147–156
Minning S et al (2001) Optimization of the high-level production of Rhizopus oryzae lipase in Pichia pastoris. J Biotechnol 86:59–70
Resina D, Serrano A, Valero F, Ferrer P (2004) Expression of a Rhizopus oryzae lipase in Pichia pastoris under control of the nitrogen source-regulated formaldehyde dehydrogenase promoter. J Biotechnol 109:103–113
Guillen M, Benaiges MD, Valero F (2011) Comparison of the biochemical properties of a recombinant lipase extract from Rhizopus oryzae expressed in Pichia pastoris with a native extract. Biochem Eng J 54:117–123
Cos O, Ramon R, Montesinos JL, Valero F (2006) Operational strategies, monitoring and control of heterologous protein production in the methylotrophic yeast Pichia pastoris under different promoters: a review. Microb Cell Fact 5:17
Mcgrew JT et al (1997) Expression of trimeric CD40 ligand in Pichia pastoris: use of a rapid method to detect high-level expressing transformants. Gene 187:193–200
Gellissen G (2000) Heterologous protein production in methylotrophic yeasts. Appl Microbiol Biotechnol 54:741–750
van der Klei IJ, Yurimoto H, Sakai Y, Veenhuis M (2006) The significance of peroxisomes in methanol metabolism in methylotrophic yeast. Biochim Biophys Acta-Mol Cell Res 1763:1453–1462
Loureiro-Dias MC (1988) Movements of protons coupled to glucose transport in yeasts. A comparative study among 248 yeast strains. Antonie Van Leeuwenhoek 54:331–343
Sibirny AA et al (1988) Genetic control of methanol utilization in yeasts. J Basic Microbiol 28:293–319
van Urk H, Postma E, Scheffers WA, van Dijken JP (1989) Glucose transport in crabtree-positive and crabtree-negative yeasts. J Gen Microbiol 135:2399–2406
Sinha J, Plantz BA, Inan M, Meagher MM (2005) Causes of proteolytic degradation of secreted recombinant proteins produced in methylotrophic yeast Pichia pastoris: case study with recombinant ovine interferon-tau. Biotechnol Bioeng 89:102–112
Zhang P et al (2010) Catabolite repression of Aox in Pichia pastoris is dependent on hexose transporter PpHxt1 and pexophagy. Appl Environ Microbiol 76:6108–6118
Waterham HR et al (1997) Isolation of the Pichia pastoris glyceraldehyde-3-phosphate dehydrogenase gene and regulation and use of its promoter. Gene 186:37–44
Garcia-Ortega X, Ferrer P, Montesinos JL, Valero F (2013) Fed-batch operational strategies for recombinant Fab production with Pichia pastoris using the constitutive GAP promoter. Biochem Eng J 79:172–181
Heyland J, Fu JA, Blank LM, Schmid A (2010) Quantitative physiology of Pichia pastoris during glucose-limited high-cell density fed-batch cultivation for recombinant protein production. Biotechnol Bioeng 107:357–368
Cregg JM, Cereghino JL, Shi J, Higgins DR (2000) Recombinant protein expression in Pichia pastoris. Mol Biotechnol 16:23–52
Acknowledgments
The authors wish to thank Mrs. Lilja Tuohimaa, Mrs. Tuula Karppinen, B.Sc Henna Karppinen, and M.Sc Ville-Hermanni Sotaniemi for their skillful technical assistance. Prof. Pau Ferrer is gratefully acknowledged for providing the Pichia pastoris strains and the anti-ROL antibody. Dr. David Resina, Dr. Monika Bollok and the other members of the ERA-IB EngBioCat consortium are thanked for many helpful discussions. This study was funded by the Finnish Funding Agency for Technology and Innovation (Tekes), decision number 40345. The project was part of ERA-IB-project implementing an enzyme engineering technology platform for the provision of tailor-made enzymes (EngBioCat). Also Finnish Foundation for Technology Promotion and Tauno Tönning Research Foundation are thanked for the financial support.
Conflict of interest
JPP, AV and PN are co-founders and minor shareholders of BioSilta Oy. AV is R&D director, and PN is a scientific advisor of the company. AM, HO and JK declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
449_2013_1098_MOESM1_ESM.tif
Supplementary Fig. 1 Western blot analysis of expressed ROL after 64 h of cultivation. Samples were taken from induced cultivations (BMEB or BSEB) made with different concentrations of the glucose-releasing enzyme (0.5, 1 or 2 U l−1), from negative control cultivation (WT) and reference cultivation with BMM medium. Equal volumes of culture supernatant were loaded to the gel. BMEB buffered minimal medium including the glucose polymer but no glycerol. BSEB BioSilta’s prototype EnBase medium version. BMM buffered minimal methanol medium. ROL was detected by using suitable antibodies and a horseradish peroxidase-coupled color reagent (a) and by ECL (b). In b, the higher concentrations of ROL were detected as white bands because of over exposure of the detection film. (TIFF 117 kb)
Rights and permissions
About this article
Cite this article
Panula-Perälä, J., Vasala, A., Karhunen, J. et al. Small-scale slow glucose feed cultivation of Pichia pastoris without repression of AOX1 promoter: towards high throughput cultivations. Bioprocess Biosyst Eng 37, 1261–1269 (2014). https://doi.org/10.1007/s00449-013-1098-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00449-013-1098-9