Abstract
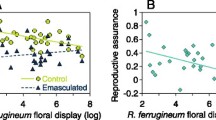
Autonomous selfing can provide reproductive assurance (RA) for flowering plants that are unattractive to pollinators or in environments that are pollen limited. Pollen limitation may result from the breakdown of once-continuous habitat into smaller, more isolated patches (habitat fragmentation) if fragmentation negatively impacts pollinator populations. Here we quantify the levels of pollen limitation and RA among large and small populations of Collinsia parviflora, a wildflower with inter-population variation in flower size. We found that none of the populations were pollen limited, as pollen-supplemented and intact flowers did not differ in seed production. There was a significant effect of flower size on RA; intact flowers (can self) produced significantly more seeds than emasculated flowers (require pollen delivery) in small-flowered plants but not large-flowered plants. Population size nested within flower size did not significantly affect RA, but there was a large difference between our two replicate populations for large-flowered, small populations and small-flowered, large populations that appears related to a more variable pollination environment under these conditions. In fact, levels of RA were strongly negatively correlated with rates of pollinator visitation, whereby infrequent visitation by pollinators yielded high levels of RA via autonomous selfing, but there was no benefit of autonomous selfing when visitation rates were high. These results suggest that autonomous selfing may be adaptive in fragmented habitats or other ecological circumstances that affect pollinator visitation rates.



Similar content being viewed by others
References
Aarssen LW (2000) Why are most selfers annuals? A new hypothesis for the fitness benefit of selfing. Oikos 89:606–612
Aguilar R, Ashworth L, Galetto L, Aizen MA (2006) Plant reproductive susceptibility to habitat fragmentation: review and synthesis through a meta-analysis. Ecol Lett 9:968–980
Armbruster WS, Mulder CPH, Baldwin BG, Kalisz S, Wessa B, Nute H (2002) Comparative analysis of late floral development and mating-system evolution in tribe Collinsieae (Scrophulariaceae S.L.). Am J Bot 89:37–49
Ashman TL, Knight TM, Steets JA, Amarasekare P, Burd M, Campbell DL, Johnston MO, Mazer SJ, Mitchell RJ, Morgan MT, Wilson WG (2004) Pollen limitation of plant reproduction: ecological and evolutionary causes and consequences. Ecology 85:2408–2421
Becerra J, Lloyd D (1992) Competition-dependent abscission of self-pollinated flowers of Phormium tenax (Agavaceae): a second action of self-incompatibility at the whole flower level. Evolution 46:458–469
Bell G (1985) On the function of flowers. Proc R Soc Lond 224: 223–265
Celedon-Neghme C, Gonzales WL, Gianoli E (2007) Cost and benefits of attractive floral traits in the annual species Madia sativa (Asteraceae). Evol Ecol 21:247–257
Cheptou P-O, Avendaño V LG (2006) Pollination processes and the Allee effect in highly fragmented populations: consequences for the mating system in urban environments. New Phytol 172:774–783
Conner J (1997) Floral evolution in wild radish: the roles of pollinators, natural selection, and genetic correlations among traits. Int J Plant Sci 158:S108–120
Cruden R, Lyon D (1985) Patterns of biomass allocation to male and female functions in plants with different mating systems. Oecologia 66:299–306
Cruden R, Lyon D (1989) Facultative xenogamy: examination of a mixed mating system. In: Bock J, Linhart Y (eds) The evolutionary ecology of plants. Westview, Boulder, pp 171–207
Culley TM (2002) Reproductive biology and delayed selfing in Viola pubescens (Violaceae), an understory herb with chasmogamous and cleistogamous flowers. Int J Plant Sci 163:113–122
Darwin C (1876) The effects of cross and self-fertilization in the vegetable kingdom. Murray, London
Day RW, Quinn GP (1989) Comparisons of treatments after an analysis of variance in ecology. Ecol Monogr 59:433–463
Diggle PK (1992) Development and the volution of plant reproductive characters. In: Wyatt R (ed) Ecology and evolution of plant reproduction. Chapman and Hall, New York, pp 326–355
Douglas GW, Meidinger DV, Pojar J (2000) Illustrated Flora of British Columbia, vol 5. Dicotyledons (Salicaceae through Zygophyllaceae) and pteridophytes. British Columbia Ministry of Environment, Lands & Parks, British Columbia Ministry of Forests, Victoria
Eckert CG, Schaefer A (1998) Does self-pollination provide reproductive assurance in Aquilegia Canadensis (Ranunculaceae)? Am J Bot 85:919–924
Eckhart VM, Geber MA (1999) Character variation and geographic distribution of Clarkia xantiana A. Gray (Onagraceae): flowers and phenology distinguish two subspecies. Madroño 46:117–125
Elle E (2004) Floral adaptations and biotic and abiotic selection pressures. In: Cronk QCB, Whitton J, Ree RH, Taylor IEP (eds) Plant adaptation: molecular genetics and ecology. Proceedings of an International Workshop, 11–13 December 2002, Vancouver, British Columbia. NRC Research Press, Ottawa, pp 111–118
Elle E, Carney R (2003) Reproductive assurance varies with flower size in Collinsia parviflora (Scrophularaceae). Am J Bot 90:888–896
Fisher RA (1941) Average excess and average effect of a gene substitution. Ann Eugen 11:53–63
Fuchs MA (2001) Towards a recovery strategy for Garry oak and associated ecosystems in Canada: ecological assessment and literature review. Technical report GBEI/EC-00-030. Environment Canada, Canadian Wildlife Service, Pacific and Yukon Region
Ganders FR, Krause GR (1986) Systematics of Collinsia parviflora and C. grandiflora (Scrophulariaceae). Madroño 33:63–70
Ghazoul J (2005) Pollen and seed dispersal and dispersed plants. Biol Rev 80:1–31
Goodwillie C, Kalisz S, Eckert CG (2005) The evolutionary enigma of mixed mating systems in plants: occurrence, theoretical explanations, and empirical evidence. Annu Rev Ecol Evol Syst 36:47–79
Guerrant EOJ (1989) Early maturity, small flowers, and autogamy: a developmental connection? In: Bock J, Linhart Y (eds) The evolutionary ecology of plants. Westview, Boulder, pp 61–84
Herlihy CR, Eckert CG (2002) Genetic cost of reproductive assurance in a self-fertilizing plant. Nature 416:320–323
Jennersten O (1988) Pollination in Dianthus deltoides (Caryophyllaceae): effects of habitat fragmentation on visitation and seed set. Conserv Biol 2:359–366
Kalisz S, Vogler DW (2003) Benefits of autonomous selfing under unpredictable pollinator environments. Ecology 84:2928–2942
Kennedy BF, Sabara HA, Haydon D, Husband BC (2006) Pollinator-mediated assortative mating in mixed ploidy populations of Chamerion angustifolium (Onagraceae). Oecologia 150:398–408
Kéry M, Matthies D (2004) Reduced fecundity in small populations of the rare plant Gentianopsis ciliate (Gentianaceae). Plant Biol 6:683–688
Kimura M (1959) Conflict between self-fertilization and outbreeding in plants. Annu Report Nat Inst Jpn 9:87–88
Knight TM, Steets JA, Vamosi JC, Mazer SJ, Burd M, Campbell DR, Dudash MR, Johnston MO, Mitchell RJ, Ashman T-L (2005) Pollen limitation of plant reproduction: pattern and process. Annu Rev Ecol Evol Syst 36:467–497
Lande R, Schemske D (1985) The evolution of self-fertilization and inbreeding depression in plants. I. Genetic models. Evolution 39:24–40
Lea T (2002) Historical Garry oak ecosystems of Greater Victoria and the Saanich Peninsula background information. Terrestrial Information Branch, British Columbia Ministry of Sustainable Resource Management, Victoria
Lloyd DG (1992) Self- and cross-fertilization in plants. II. The selection of self-fertilization. Int J Plant Sci 153:370–380
Moeller DA (2004) Facilitative interactions among plants via shared pollinators. Ecology 85:3289–3301
Moeller DA, Geber MA (2005) Ecological context of the evolution of self-pollination in Clarkia xantiana: population size, plant communities, and reproductive assurance. Evolution 59:786–799
Moeller DA (2006) Geographic structure of pollinator communities, reproductive assurance, and the evolution of self-pollination. Ecology 87:1510–1522
Morgan MT, Wilson WG (2005) Self-fertilization and the escape from pollen limitation in variable pollination environments. Evolution 59:1143–1148
Palmer TM, Stanton ML, Young TP (2003) Competition and coexistence: exploring mechanisms that restrict and maintain diversity within mutualist guilds. Am Nat 162:S63–S79
Parachnowitsch AL, Elle E (2004) Variation in sex allocation and male-female trade-offs in sex populations of Collinsia parviflora (Scrophulariaceae S.L.). Am J Bot 91: 1200–1207
Runions CJ, Geber MA (2000) Evolution of the self-pollinating flower in Clarkia xantiana (Onagraceae). I. Size and development of floral organs. Am J Bot 87:1439–1451
SAS (1996) SAS/STAT software: changes and enhancements for release, version 6.12. SAS Institute, Cary
Schoen DJ, Morgan MT, Bataillon T (1996) How does self-pollination evolve? Inferences from floral ecology and molecular genetic variation. Philos Trans R Soc Lond B Biol Sci 351:1281–1290
Sih A, Baltus M (1987) Patch size, pollinator behaviour, and pollinator limitation in catnip. Ecology 68:1679–1690
Stebbins GL (1957) Self-fertilization and population variability in the higher plants. Am Nat 91:337–354
Steffan-Dewenter I, Tscharntke T (1999) Effects of habitat isolation on pollinator communities and seed set. Oecologia 121:432–440
Thompson JD (2001) How do visitation patterns vary among pollinators in relation to floral display and floral design in a generalist pollination system? Oecologia 126:386–394
Vogler DW, Kalisz S (2001) Sex among flowers: the distribution of plant mating systems. Evolution 55:202–204
Wagenius S (2006) Scale dependence of reproductive failure in fragmented Echinacea populations. Ecology 87:931–941
Wilcock C, Neiland R (2002) Pollination failure in plants: why it happens and when it matters. Trends Plant Sci 7:270–276
Acknowledgements
We thank E. Fairhurst, S. Gillespie, and E. Jones for field assistance; J. Conner, D. Moeller, S. Kalisz, C. Herlihy, M. Hart, D. Green, and an anonymous reviewer for helpful conversations and/or constructive comments on the manuscript; I. Bercovitz and C. Schwarz for statistical advice; and Capital Regional District Parks, The Nature Conservancy of Canada, British Columbia Provincial Parks, District of Saanich Parks, District of Esquimalt Parks, and TimberWest for access to field sites. This research was supported by a Discovery Grant to E. Elle from the Natural Sciences and Engineering Research Council of Canada. All research complied with the current regulations of landowners and the Canadian Government.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jeff Conner.
Rights and permissions
About this article
Cite this article
Kennedy, B.F., Elle, E. The reproductive assurance benefit of selfing: importance of flower size and population size. Oecologia 155, 469–477 (2008). https://doi.org/10.1007/s00442-007-0924-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00442-007-0924-7