Abstract
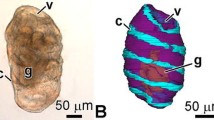
The development of respiratory trees in the holothurian Apostichopus japonicus has been studied using light and electron microscopy. Primordial respiratory trees emerge in 2–3-mm-long animals (2 months after fertilization). They arise as two independent outgrowths from the dorsal wall of the anterior part of the cloaca. Upon first emerging and throughout the course of development, the left respiratory tree is longer than the right one. A common base develops in 4-mm-long animals (2–3 months after fertilization). In yearlings, the left respiratory tree grows into gaps between the loops of the intestinal tube interlaced with intestinal hemal vessels. The develo** coelomic and luminal epithelia have ultrastructural peculiarities. The luminal epithelium of respiratory trees has been shown for the first time to comprise nerve cells and their processes. Characteristic structural features of the epithelia are shown to begin develo** as early as in 4-mm-long animals (2–3 months after fertilization). In yearlings, the respiratory trees demonstrate definitive structural patterns and are entirely functional.






Similar content being viewed by others
References
Banerjee S, Sousa AD, Bhat MA (2006) Organization and function of septate junction. Cell Biochem Biophys 46:65–77
Bely AE, Nyberg KG (2010) Evolution of animal regeneration: re-emergence of a field. Trends Ecol Evol 25(3):161–170
Brockes JP (1998) Progenitor cells for regeneration: origin by reversal of the differentiated state. In: Ferretti P, Géraudie J (eds) Cellular and molecular basis of regeneration: from invertebrates to humans. John Wiley & Sons, Chichester, pp 63–77
Brockes JP, Kumar A (2008) Comparative aspects of animal regeneration. Ann Review Cell Develop Biol 24:525–549
Carlson BM (1998) Development and regeneration, with special emphasis on the amphibian limb. In: Ferretti P, Géraudie J (eds) Cellular and molecular basis of regeneration: from invertebrates to humans. John Wiley & Sons, Chichester, pp 45–62
Dolmatov IYu (1999) Regeneration in echinoderms. Russian J Marine Biol 25:225–233
Dolmatov IYu, Ginanova TT (2009) Post-autotomy regeneration of the respiratory trees in the holothurian Apostichopus japonicus (Holothurioidea, Aspidochirotida). Cell Tissue Res 336:41–58
Dolmatov IYu, Ivantey VA (1993) Histogenesis of longitudinal muscle bands in holothurians. Russian J Develop Biol 24:67–72
Dolmatov IYu, Mashanov VS (2007) Regeneration in holothurians. Dalnauka, Vladivostok
Dolmatov IYu, Mokretsova ND (1995) Morphology of pentactula of Cucumaria japonica (Dendrochirota, Holothuroidea). Zoologicheskiy Zhurnal 74(1):83–91
Dolmatov IYu, Yushin VV (1993) Larval development of Eupentacta fraudatrix (Holothuroidea, Dendrochirota). Asian Marine Biol 10:125–134
Dolmatov IYu, Eliseikina MG, Bulgakov AA, Ginanova TT, Lamash NE, Korchagin VP (1996) Muscle regeneration in the holothurian Stichopus japonicus. Roux’s Archive Develop Biol 205:486–493
Dolmatov IYu, Mashanov VS, Zueva OR (2007) Derivation of muscles of the Aristotle’s lantern from coelomic epithelia. Cell Tissue Res 327:371–384
Dolmatov IYu, Nguyen An Khang, Kamenev YaO (2012) Preliminary data about of asexual reproduction, evisceration, and regeneration in holothurians of Nha Trang Bay (Vietnam, South China Sea). Russian J Marine Biol, in press
Doyle WL, McNeill GF (1964) The fine structure of the respiratory tree in Cucumaria. Q J Microsc Sci 105:7–11
Farmanfarmaian A (1966) The respiratory physiology of echinoderms. In: Boolootian RA (ed) Physiology of echinodermata. Wiley Interscience, New York, pp 245–266
Feral JP, Massin C (1982) Digestive system: Holothuroidea. In: Jangoux M, Lawrence JM (eds) Echinoderm nutrition. Balkema, Rotterdam, pp 192–212
García-Arrarás JE, Dolmatov IYu (2010) Echinoderms: potential model systems for studies on muscle regeneration. Cur Pharmac Des 16:942–955
García-Arrarás JE, Díaz-Miranda L, Torres II, File S, Jiménez LB, Rivera-Bermudez K, Arroyo EJ, Cruz W (1999) Regeneration of the enteric nervous system in the sea cucumber Holothuria glaberrima. J Comp Neurol 406:461–475
Humason GL (1962) Animal tissue techniques. WH Freeman and Company, San Francisco
Hyman LH (1955) The invertebrates: Echinodermata. The coelome Bilateria. McGraw-Hill, New York
Levin VS (1982) Japanese sea cucumber. Vladivostok Publisher, Vladivostok
Levin VS (2000) Japanese sea cucumber: biology, fisheries, cultivation. Goland, Saint Petersburg
Malakhov VV, Cherkasova IV (1991) The embryonal and early development of Stichopus japonicus var. armatus (Aspidochirota, Stichopodidae). Zoologitchesky Zhurnal 70:55–67
Malakhov VV, Cherkasova IV (1992) Metamorphosis of the sea cucumber Stichopus japonicus (Aspidochirota, Stichopodidae). Zoologitchesky Zhurnal 71:11–21
Mashanov VS, Dolmatov IYu (2000) Developmental morphology of a holothurian, Cucumaria japonica (Dendrochirota, Holothuroidea), a species with accelerated metamorphosis. Invertebrate Reprod Develop 37:137–146
Mashanov VS, Frolova LT, Dolmatov IYu (2004) Structure of the digestive tube in the holothurian Eupentacta fraudatrix (Holothuroidea, Dendrochirota). Russian J Marine Biol 30:314–322
Murray G, García-Arrarás JE (2004) Myogenesis during holothurian intestinal regeneration. Cell Tissue Res 318:515–524
Nørrevang A, Winstrand KG (1970) On the occurrence and structure of choanocyte-like cells in some echinoderms. Acta Zool 51:249–270
Robertson DA (1972) Volume changes and oxygen extraction efficiency in the holothurian Stichopus mollis. Comp Biochem Physiol 43:795–800
Sisak MM, Sander F (1985) Respiratory behaviour of the western Atlantic holothuroidean, at various salinities, temperatures and oxygen tensions. Comp Biochem Physiol 80A:25–30
Smiley S (1994) Holothuroidea. In: Harrison FW, Chia FS (eds) Microscopic anatomy of invertebrates, vol 14: Echinodermata. Wiley-Liss Inc, New York, pp 401–471
Smiley S, McEuen FS, Chaffee C, Krishnan S (1991) Echinodermata: Holothuroidea. In: Gies AC, Pearse JS, Pearse VB (eds) Reproduction of marine invertebrates, vol 6. Boxwood, Pacific Grove CA, pp 633–750
Spirina IS, Dolmatov IYu (2001) Respiratory tree morphology of holothurians Apostichopus japonicus and Cucumaria japonica. Russian J Marine Biol 27:421–429
Spirina IS, Dolmatov IYu (2003) Mitotic activity in tissues of the regenerating respiratory tree of the holothurian Apostichopus japonicus (Holothuroidea, Aspidochirota). Russian J Marine Biol 29:123–125
Torelle E (1910) Regeneration in Holothuria. Zool Anz 35:15–22
Wolcott TG (1981) Inhaling without ribs: the problem of suction in soft-bodied invertebrates. Biol Bull 160:189–197
Acknowledgments
We are grateful to the anonymous reviewers, whose valuable critical comments enabled us to improve the quality of the manuscript. Our special thanks are extended to D.A. Andreev and A.V. Korneichuk for technical assistance. This study was supported in part by the Far Eastern Branch of Russian Academy of Sciences (grant № 09-I-P22-02), Russian Foundation for Basic Research (grant № 11-04-00408) and Russian Government (grant № 11.G34.31.0010).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dolmatov, I.Y., Frolova, L.T., Zakharova, E.A. et al. Development of respiratory trees in the holothurian Apostichopus japonicus (Aspidochirotida: Holothuroidea). Cell Tissue Res 346, 327–338 (2011). https://doi.org/10.1007/s00441-011-1280-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-011-1280-9