Abstract
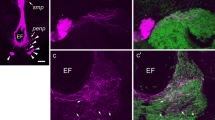
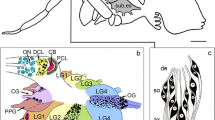
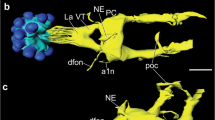
Antisera against a variety of vertebrate and invertebrate neuropeptides were used to characterize neurons with somata in the pars intercerebralis (PI), pars lateralis (PL), and subesophageal ganglion (SEG), designated as PI neurons, PL neurons, and SEG neurons, respectively, all of which project to the retrocerebral complex in the blow fly, Protophormia terraenovae. Immunocytochemistry combined with backfills through the cardiac-recurrent nerve revealed that at least two pairs of PI and SEG neurons for each were FMRFamide-immunoreactive. Immunoreactivity against [Arg7]-corazonin, β-pigment-dispersing hormone (β-PDH), cholecystokinin8, or FMRFamide was observed in PL neurons. Immunoreactive colocalization of [Arg7]-corazonin with β-PDH, [Arg7]-corazonin with cholecystokinin8, or β-PDH with FMRFamide was found in two to three somata in the PL of a hemisphere. Based on their anatomical and immunocytochemical characteristics, PI neurons were classified into two types, PL neurons into six types, and SEG neurons into two types. Fibers in the retrocerebral complex showed [Arg7]-corazonin, β-PDH, cholecystokinin8, and FMRFamide immunoreactivity. Cholecystokinin8 immunoreactivity was also detected in intrinsic cells of the corpus cardiacum. The corpus allatum was densely innervated by FMRFamide-immunoreactive varicose fibers. These results suggest that PI, PL, and SEG neurons release [Arg7]-corazonin, β-PDH, cholecystokinin8, or FMRFamide-like peptides from the corpus cardiacum or corpus allatum into the hemolymph, and that some PL neurons may simultaneously release several neuropeptides.





Similar content being viewed by others
References
Agui N, Bollenbacher WE, Granger NA, Gilbert LI (1980) Corpus allatum is release site for insect prothoracicotropic hormone. Nature 285:6669–6670
Brown MR, Lea AO (1988) FMRFamide- and adipokinetic hormone-like immunoreactivity in the nervous system of the mosquito, Aedes aegypti. J Comp Neurol 270:606–614
Brown MR, Graf R, Swiderek KM, Fendley D, Stracker TH, Champagne DE, Lea AO (1998) Identification of a steroidogenic neurohormone in female mosquitoes. J Biol Chem 273:3967–3971
Cantera R, Veenstra JA, Nässel DR (1994) Postembryonic development of corazonin-containing neurons and neurosecretory cells in the blowfly, Phormia terraenovae. J Comp Neurol 350:559–572
Cao C, Brown MR (2001) Localization of an insulin-like peptide in brains of two flies. Cell Tissue Res 304:317–321
Choi YJ, Lee G, Hall JC, Park JH (2005) Comparative analysis of corazonin-encoding genes (crzs) in Drosophila species and functional insights into crz-expressing neurons. J Comp Neurol 482:372–385
Davis NT (1985) Serotonin-immunoreactive visceral nerves and neurohemal system in the cockroach Periplaneta americana (L.). Cell Tissue Res 240:593–600
Dircksen H, Zahnow CA, Gaus G, Keller R, Rao KR, Riehm JP (1987) The ultrastructure of nerve endings containing pigment-dispersing hormone (PDH) in crustacean sinus glands: identification by an antiserum against a synthetic PDH. Cell Tissue Res 250:377–387
Duve H, Thorpe A (2003) Neuropeptide co-localization in the lepidopteran frontal ganglion studied by confocal laser scanning microscopy. Cell Tissue Res 311:79–89
Duve H, Thorpe A, Scott AG, Johnsem AH, Rehfeld JF, Hines E, East PD (1995) The sulfakinins of the blowfly Calliphora vomitoria: peptide isolation, gene cloning and expression studies. Eur J Biochem 232:633–640
Eckert M, Ude J (1983) Immunocytochemical techniques for the identification of peptidergic neurons. In: Strausfeld NJ (ed) Functional neuroanatomy. Springer, Berlin Heidelberg New York, pp 267–301
Hamanaka Y, Numata H, Shiga S (2004) Morphology and electrical properties of neurons projecting to the retrocerebral complex in the blow fly, Protophormia terraenovae. Cell Tissue Res 318:403–418
Hamanaka Y, Yasuyama K, Numata H, Shiga S (2005) Synaptic connections between pigment-dispersing factor-immunoreactive neurons and neurons in the pars lateralis of the blow fly Protophormia terraenovae. J Comp Neurol 491:390–399
Homberg U, Davis NT, Hildebrand JG (1991) Peptide-immunocytochemistry of neurosecretory cells in the brain and retrocerebral complex of the sphinx moth Manduca sexta. J Comp Neurol 303:35–52
Ichikawa T (2003) Firing activities of neurosecretory cells producing diapause hormone and its related peptides in the female silkmoth, Bombyx mori. I. Labial cells. Zool Sci 20:971–978
Ichikawa T, Hasegawa K, Shimizu I, Katsuno K, Kataoka H, Suzuki A (1995) Structure of neurosecretory cells with immunoreactive diapause hormone and pheromone biosynthesis activating neuropeptides in the silkmoth, Bombyx mori. Zool Sci 12:703–712
Ichikawa T, Shiota T, Shimizu I, Kataoka H (1996) Functional differentiation of neurosecretory cells with immunoreactive diapause hormone and pheromone biosynthesis activating neuropeptide of the moth, Bombyx mori. Zool Sci 13:21–25
Kim Y-J, Spalovská-Valachová I, Cho K-H, Zitnanova I, Park Y, Adams ME, Žitňan D (2004) Corazonin receptor signaling in ecdysis initiation. Proc Natl Acad Sci USA 101:6704–6709
Lee G, Bahn JH, Park JH (2006) Sex- and clock-controlled expression of the neuropeptide F gene in Drosophila. Proc Natl Acad Sci USA 103:12580–12585
Lin H, Yin C-M, Stoffolano JG Jr, Garofalo RS (2005) Immunological localization of mosquito ovary ecdysteroidogenic hormone I and fruit fly insulin receptor in adult Phormia regina (Diptera: Calliphoridae). Physiol Biochem Toxicol 98:329–335
Lloyd GL, Woodhead AP, Stay B (2000) Release of neurosecretory granules within the corpus allatum in relation to the regulation of juvenile hormone synthesis in Diploptera punctata. Insect Biochem Mol Biol 30:739–746
Manière G, Rondot I, Büllesbach EE, Gautron F, Vanhems E, Delbecque JP (2004) Control of ovarian steroidogenesis by insulin-like peptides in the blowfly (Phormia regina). J Endocrinol 181:147–156
Matsuo J, Shiga S, Numata H (1997) Role of the corpus allatum in the control of adult diapause in the blow fly, Protophormia terraenovae. J Insect Physiol 43:211–216
McCormick J, Nichols R (1993) Spatial and temporal expression identify dromyosuppressin as a brain-gut peptide in Drosophila melanogaster. J Comp Neurol 338:279–288
Mizoguchi A, Ishizaki H, Nagasawa H, Kataoka H, Isogai A, Tamura S, Suzuki A, Fu**o M, Kitada C (1987) A monoclonal antibody against a synthetic fragment of bombyxin (4K-prothoracicotropic hormone) from the silkmoth, Bombyx mori: characterization and immunohistochemistry. Mol Cell Endocrinol 51:227–235
Mizoguchi A, Oka T, Kataoka H, Nagasawa H, Suzuki A, Ishizaki H (1990) Immunohistochemical localization of prothoracicotropic hormone-producing neurosecretory cells in the brain of Bombyx mori. Dev Growth Differ 32:591–598
Nässel DR (2002) Neuropeptides in the nervous system of Drosophila and other insects: multiple roles as neuromodulators and neurohormones. Prog Neurobiol 68:1–84
Nässel DR, Shiga S, Mohrherr CJ, Rao KR (1993) Pigment-dispersing hormone-like peptide in the nervous system of the flies Phormia and Drosophila: immunocytochemistry and partial characterization. J Comp Neurol 331:183–198
Nichols R, Lim IA (1996) Spatial and temporal immunocytochemical analysis of drosulfakinin (Dsk) gene products in the Drosophila melanogaster central nervous system. Cell Tissue Res 283:107–116
Nichols R, Schneuwly SA, Dixon JE (1988) Identification and characterization of a Drosophila homologue to the vertebrate neuropeptide cholecystokinin. J Biol Chem 263:12167–12170
Numata H, Shiga S (1995) Induction of adult diapause by photoperiod and temperature in Protophormia terraenovae (Diptera: Calliphoridae) in Japan. Environ Entomol 24:1633–1636
O’Brien MA, Katahira EJ, Flanagan TR, Arnold LW, Haughton G, Bollenbacher WE (1988) A monoclonal antibody to the insect prothoracicotropic hormone. J Neurosci 8:3247–3257
O’Brien MA, Schneider LE, Taghert PH (1991) In situ hybridization analysis of the FMRFamide neuropeptide gene in Drosophila. II. Constancy in the cellular pattern of expression during metamorphosis. J Comp Neurol 304:623–638
Orchard I, Loughton BG (1985) Neurosecretion. In: Kerkut GA, Gilbert LI (eds) Comprehensive insect physiology, biochemistry and pharmacology, vol 7. Endocrinology I. Pergamon, Oxford, pp 61–107
Orchard I, Lange AB, Bendena WG (2001) FMRFamide-related peptides: a multifunctional family of structurally related neuropeptides in insects. Adv Insect Physiol 28:267–329
Predel R, Wegener C, Russell WK, Tichy SE, Russell DH, Nachman RJ (2004) Peptidomics of CNS-associated neurohemal system of adult Drosophila melanogaster: a mass spectrometric survey of peptides from individual flies. J Comp Neurol 474:379–392
Raabe M (1989) Recent developments in insect neurohormones. Plenum, New York
Raikhel AS, Brown MR, Belles X (2005) Hormonal control of reproductive process. In: Gilbert LI, Iatrou K, Gill SS (eds) Comprehensive molecular insect science, vol 3. Endocrinology. Elsevier, Amsterdam, pp 433–491
Rajashekhar KP, Singh RN (1994) Neuroarchitecture of the tritocerebrum of Drosophila melanogaster. J Comp Neurol 349:633–645
Rao KR, Riehm JP (1993) Pigment-dispersing hormones. Ann NY Acad Sci 680:78–88
Renn SCP, Park JH, Rosbash M, Hall JC, Taghert PH (1999) A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell 99:791–802
Richer S, Stoffolano JG Jr, Yin C-M, Nichols R (2000) Innervation of dromyosuppressin (DMS) immunoreactive processes and effect of DMS and benzethonium chloride on the Phormia regina (Meigen) crop. J Comp Neurol 421:136–142
Schneider LE, Taghert PH (1988) Isolation and characterization of a Drosophila gene that encodes multiple neuropeptides related to Phe-Met-Arg-Phe-NH2 (FMRFamide). Proc Natl Acad Sci USA 85:1993–1997
Schneider LE, Sun ET, Garland DJ, Taghert PH (1993) An immunocytochemical study of the FMRFamide neuropeptide gene products in Drosophila. J Comp Neurol 337:446–460
Shiga S, Numata H (2000) The roles of neurosecretory neurons in the pars intercerebralis and pars lateralis in reproductive diapause of the blow fly, Protophormia terraenovae. Naturwissenschaften 87:125–128
Shiga S, Toyoda I, Numata H (2000) Neurons projecting to the retrocerebral complex of the adult blow fly, Protophormia terraenovae. Cell Tissue Res 299:427–439
Shiga S, Davis NT, Hildebrand JG (2003) Role of neurosecretory cells in the photoperiodic induction of pupal diapause of the tobacco hornworm Maduca sexta. J Comp Neurol 462:275–285
Siegmund T, Korge G (2001) Innervation of the ring gland of Drosophila melanogaster. J Comp Neurol 431:481–491
Stay B, Chan KK, Woodhead AP (1992) Allatostatin-immunoreactive neurons projecting to the corpora allata of adult Diploptera punctata. Cell Tissue Res 270:15–23
Strand FL (1999) Neuropeptides: regulators of physiological processes. MIT, Cambridge
Tanaka Y, Hua Y-J, Roller L, Tanaka S (2002) Corazonin reduces the spinning rate in the silkworm, Bombyx mori. J Insect Physiol 48:707–714
Toyoda I, Shiga S, Numata H (1999) Role of the median neurosecretory cells in the ovarian development of the blow fly Protophormia terraenovae. Zool Sci 16:187–191
Veenstra JA, Davis NT (1993) Localization of corazonin in the nervous system of the cockroach Periplaneta americana. Cell Tissue Res 274:57–64
Vilim FS, Price DA, Lesser W, Kupfermann I, Weiss KR (1996) Costorage and corelease of modulatory peptide cotransmitters with partially antagonistic actions on the accessory radula closer muscle of Aplysia californica. J Neurosci 16:8092–8104
Vilim FS, Cropper EC, Price DA, Kupfermann I, Weiss KR (2000) Peptide cotransmitter release from motorneuron B16 in Aplysia californica: costorage, corelease, and functional implications. J Neurosci 20:2036–2042
Weber E, Evans CJ, Samuelsson SJ, Barchas JD (1981) Novel peptide neuronal system in rat brain and pituitary. Science 214:1248–1251
Weiss KR, Brezina V, Cropper EC, Hooper SL, Miller MW, Probst WC, Vilim FS, Kupfermann I (1992) Peptidergic co-transmission in Aplysia: functional implications for rhythmic behaviors. Experientia 48:456–463
White K, Hurteau T, Punsal P (1986) Neuropeptide-FMRFamide-like immunoreactivity in Drosophila: development and distribution. J Comp Neurol 247:430–438
Wise S, Davis NT, Tyndale E, Noveral J, Folwell MG, Bedian V, Emery IF, Siwicki KK (2002) Neuroanatomical studies of period gene expression in the hawkmoth, Manduca sexta. J Comp Neurol 447:366–380
Yamashita O (1996) Diapause hormone of the silkmoth, Bombyx mori: structure, gene expression and function. J Insect Physiol 42:669–679
Yu CG, Stay B, Joshi S, Tobe SS (1993) Allatostatin content of brain, corpora allata and haemolymph at different developmental stages of the cockroach, Diploptera punctata: quantitation by ELISA and bioassay. J Insect Physiol 39:111–122
Acknowledgements
We are grateful to Dr. N.T. Davis (University of Arizona) for reading through the manuscript and giving critical comments and for a gift of antisera. We also thank Dr. J. A. Veenstra (Université Bordeaux), Dr. M.R. Brown (University of Georgia), Dr. H. Dircksen (University of Bonn), Dr. A. Mizoguchi (Nagoya University), Dr. D.R. Nässel (Stockholm University), and Dr. B. Stay (University of Iowa) for their generous gifts of antisera.
Author information
Authors and Affiliations
Corresponding author
Additional information
This work was supported by a Grant-in-Aid for Scientific Research C (17570065) from the Japan Society for the Promotion of Science (to S.S.) and a Grant-in-Aid for Scientific Research B (16370038) from the Japan Society for the Promotion of Science (to H.N.).
Rights and permissions
About this article
Cite this article
Hamanaka, Y., Tanaka, S., Numata, H. et al. Peptide immunocytochemistry of neurons projecting to the retrocerebral complex in the blow fly, Protophormia terraenovae . Cell Tissue Res 329, 581–593 (2007). https://doi.org/10.1007/s00441-007-0433-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00441-007-0433-3