Abstract
Alternative pre-mRNA splicing is a tightly controlled process conducted by the spliceosome, with the assistance of several regulators, resulting in the expression of different transcript isoforms from the same gene and increasing both transcriptome and proteome complexity. The differences between alternative isoforms may be subtle but enough to change the function or localization of the translated proteins. A fine control of the isoform balance is, therefore, needed throughout developmental stages and adult tissues or physiological conditions and it does not come as a surprise that several diseases are caused by its deregulation. In this review, we aim to bring the splicing machinery on stage and raise the curtain on its mechanisms and regulation throughout several systems and tissues of the human body, from neurodevelopment to the interactions with the human microbiome. We discuss, on one hand, the essential role of alternative splicing in assuring tissue function, diversity, and swiftness of response in these systems or tissues, and on the other hand, what goes wrong when its regulatory mechanisms fail. We also focus on the possibilities that splicing modulation therapies open for the future of personalized medicine, along with the leading techniques in this field. The final act of the spliceosome, however, is yet to be fully revealed, as more knowledge is needed regarding the complex regulatory network that coordinates alternative splicing and how its dysfunction leads to disease.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Consider a magic trick, one that spans millions and millions of years, performed by a world-class magician known as the spliceosome. From a single gene, multiple RNA products emerge. The results are intriguing: some of these transcripts are almost identical, and others are so unique as to exert antagonising functions. However, the trick is straightforward: it is a simple unit rearrangement of the gene sequence. However, how is such a simple trick performed? Let us unravel the magic of alternative splicing.
Alternative splicing (AS) was first reported in 1977 by the laboratories of Richard Roberts and Philip Sharp, who observed that mammalian cells infected with adenovirus 2 in lytic stage produce mRNA sequences complementary to non-contiguous DNA segments, as confirmed by electron microscopic visualisation of these alternative transcripts hybridised with single-stranded fragments of the viral genome (Berget et al. 1977; Chow et al. 1977). In the following year, Walter Gilbert suggested naming the segments included in and excluded from the mature mRNAs as “exons” and “introns”, respectively (Gilbert 1978).
Splicing in endogenous genes was revealed in the beginning of the 1980s with the findings of calcitonin and immunoglobulin alternative transcripts in mammals (Liu et al. 1980; Tucker et al. 1980; Early et al. 1980a; Rosenfeld et al. 1981, 1982). The contrasting levels of calcitonin expression in rat medullary thyroid carcinoma lines were discovered to be related with alternative transcripts later observed to originate from the same gene and to encode different proteins (Rosenfeld et al. 1981, 1982).
In addition, in the early 1980 s, the interplay between pre-mRNAs and the U1, U2, U4, U5, and U6 small nuclear ribonucleoproteins (snRNPs) started to be discussed (Lerner et al. 1980; Ohshima et al. 1981; Krainer and Maniatis 1985). These snRNPs are core components of a large ribonucleoprotein complex required for pre-mRNA splicing, known as the spliceosome (Brody and Abelson 1985; Butcher and Brow 2005), that recognises introns through cis elements present at exon–intron boundaries (5′ and 3′ splice sites) and within introns (branch point sequence and polypyrimidine tract) (Reed and Maniatis 1985; Chiou and Lynch 2014; Wongpalee and Sharma 2014). As first detailed in 1984, pre-mRNA splicing starts with the spliceosome-catalysed cleavage of the phosphodiester bond at the 5′ exon–intron junction (5′ splice site) performed by a branch point adenosine. This reaction forms an intermediary lariat structure that is subsequently liberated by the cleavage of the phosphodiester bond at the 3′ exon–intron junction (3′ splice site) performed by the free hydroxyl group of the 5′ exon, resulting in the joining of the two exons (Ruskin et al. 1984; Padgett et al. 1984; Domdey et al. 1984; Wongpalee and Sharma 2014) (see Fig. 1).
Spliceosome assembly and splicing reactions. (1) U1 snRNP binds to the 5′ splice site (5′ss), whereas the splicing factor 1 (SF1) and U2AF proteins bind to the branch point site (BPS), the polypyrimidine tract (PPT), and 3′ splice site (3′ss). The interaction between U1 and U2 snRNPs results in the formation of the pre-spliceosome. (2) The first splicing reaction is performed after the recruitment of the U4/5/6 snRNPs through a nucleophilic attack from the adenosine in the BPS to the 5′ss of the upstream exon. (3) The intron lariat is then formed. The free 3′ hydroxyl group performs a nucleophilic attack to the phosphate of the 3′ splice site of the downstream exon. (4) Finally, the intron lariat is released and both exons are ligated
AS is regulated through exonic and intronic cis-acting regions called exonic/intronic splicing enhancers or silencers that are targeted by RNA-binding proteins (RBPs) (Coelho and Smith 2014). These have been described as trans-acting splicing regulators and they have been divided in heterogeneous nuclear ribonucleoprotein particles (hnRNPs, Gallinaro et al. 1981), serine-arginine-rich proteins (SR proteins)—for instance, SRSF1 (Krainer and Maniatis 1985; Krämer and Keller 1985; Krainer et al. 1990), and auxiliary proteins—such as SF1 (Krainer and Maniatis 1985) and U2AF (Ruskin et al. 1988). Those RBPs may promote or inhibit AS or even present opposing regulatory activity depending on their binding sites’ location, as illustrated in Fig. 2 (Goren et al. 2006; Coelho and Smith 2014). Other factors that are relevant for alternative transcripts to be produced include the relative location of cis elements, the secondary structure of the pre-mRNAs, sequence modifications (such as those resulting from RNA editing), and epigenetic changes (DNA and RNA methylation, chromatin structure and histone modifications, RNA interference, etc) (Coelho and Smith 2014). The molecular mechanisms of spliceosomal assembly and pre-mRNA splicing are further detailed in the following reviews: (De Conti et al. 2013; Chiou and Lynch 2014; Coelho and Smith 2014; Matera et al. 2014; Sperling 2016).
AS regulation by RNA-binding splicing factors. Binding of specific splicing factors (SF) to intronic or exonic splicing enhancers (ISE and ESE, respectively) promotes the inclusion of the alternative exon, whereas binding of given splicing factors to intronic or exonic splicing silencers (ISS and ESS, respectively) inhibits the splicing of the alternative exon
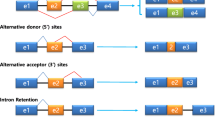
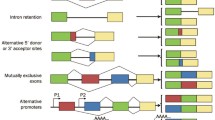
AS may occur in different manners: exon skip**, intron retention, mutually exclusive exons, alternative first and last exons, alternative 5′ and 3′ splice sites, and alternative “tandem” 5′ and 3′ untranslated regions (UTRs) (Wang et al. 2008; Wagner and Berglund 2014). However, this strict categorisation of AS events may not allow to capture the landscape of more complex AS events (Sammeth et al. 2008).
More recently, the ever-improving development and economical feasibility of genome and transcriptome sequencing have facilitated experiments providing novel insights into the physiological relevance of AS across species and tissues. Such experiments have revealed a higher number of alternatively spliced genes and AS events per gene in birds and mammals when compared to taxonomic groups with fewer cell types, suggesting a link between AS and complexity (Chen et al. 2014). Vertebrates indeed display a tissue-dependent regulation of AS splicing (Blencowe 2006; Barbosa-Morais et al. 2012; Merkin et al. 2012) and 86–88% of human protein-coding genes are reported to undergo AS (Wang et al. 2008; Chen et al. 2014).
AS promotes transcriptome diversity and is reported to be responsible for autophagy and apoptosis regulation and changes in transcription factors, protein localisation signals, protein domains (for instance, binding domain changes that alter protein interactions) and enzymatic properties (such as inactivation or activity modulation of the enzymatic core), among others (Kelemen et al. 2013; Paronetto et al. 2016). In the same line of evidence, several functions appear to be compromised upon dysregulation of AS in multiple human diseases (Tollervey et al. 2011; Oltean and Bates 2014; Paronetto et al. 2016), as a possible result of changes in cis (for instance, through mutations or single nucleotide variants—SNVs) or trans-acting regulatory elements (through alterations in their expression or protein structure, also potentially caused by SNVs) (Cartegni et al. 2002).
Aside from AS, there are other transcriptional and post-transcriptional mechanisms that regulate gene expression, such as RNA editing and RNA interference. Particularly in primates, a common element between some of these regulation mechanisms is Alu elements, the most abundant transposable sequences in humans (Häsler and Strub 2006; Jeck et al. 2013). Alu elements contain cryptic splice sites that promote exonisation and are reported to more commonly become flanking alternative exons than constitutive exons (Lev-Maor et al. 2008; Jeck et al. 2013). Moreover, intronic Alu elements may regulate AS by shifting splicing patterns through secondary structure changes to pre-mRNAs. These Alu sequences are also usual targets for RNA editing, reported to modify the conserved splice sites required for intron recognition in pre-mRNAs (Rueter et al. 1999). Interestingly, the knockdown of the RNA-editing ADAR1 enzyme in human cells leads to a significant upregulation of circular RNA expression (Ivanov et al. 2015) that compete with AS for the spliceosome recruitment, as this is required for circular RNA formation (Ashwal-Fluss et al. 2014).
The identification of genome-wide RNA–protein interactions, along with RNAi screens (Moore et al. 2010), have allowed to study splicing-regulatory networks associated with specific RBPs through high-throughput sequencing of RNA isolated by crosslinking immunoprecipitation (HITS-CLIP, also known as CLIP-Seq) (Licatalosi et al. 2008) or higher resolution, single-nucleotide CLIP-based techniques followed by high-throughput sequencing, such as iCLIP-Seq (Rossbach et al. 2014) and PAR-CliP (Hafner et al. 2010). These technologies allow to sequence RNAs targeted by an RBP of interest and have already been used to map, in mouse brains, the RNA–protein-binding sites of key splicing regulators such as Nova (Licatalosi et al. 2008), Rbfox (Weyn-Vanhentenryck et al. 2014), and Ptbp2 (Licatalosi et al. 2012). For instance, Ptbp2 has been shown to inhibit multiple adult-specific alternative exons in murine brains (Licatalosi et al. 2012), and more recently, it has been reported that the exclusion of exon 9 in human PTBP2-paralog PTBP1 alters the regulatory activity of approximately 1500 AS events (Gueroussov et al. 2015).
These and other mechanisms of regulation of AS have been extensively studied in different physiological and disease contexts, revealing an additional level of tissue-specific post-transcriptional control characterised by tight spatio-temporal modulation of gene expression. This review will elaborate on the critical contribution of the AS program to the high levels of transcriptomic complexity and functional specificity in human development and physiology. Moreover, it approaches AS changes in the context of host–pathogen interactions, neurodegeneration, cancer, and other pathological conditions. Finally, it concludes with a discussion on therapeutic approaches targeting AS.
Alternative splicing in the nervous system
The human brain contains over a trillion neurons that are connected to each other through highly specific and convoluted patterns of synaptic connections (Zaghlool et al. 2014). Due to its intrinsic complexity, the mammalian nervous system has evolved to generate a vast protein diversity by the extensive use of AS (Ule et al. 2006). In fact, AS is more abundant in the brain, comparing with other organs (Barbosa-Morais et al. 2012). Moreover, the nervous system has a specific way of regulating its AS programme based on the observations that some RBPs are uniquely expressed in neuronal populations, suggesting that they may control cell type and synapse-specific functions (Traunmüller et al. 2016). Therefore, AS in the brain must be tightly regulated, since the slightest change in splicing outputs can have profound effects in several important neuronal aspects such as neurogenesis and synaptic function (Lipscombe and Diane 2005).
During neurogenesis, AS patterns vary considerably. In the onset of neuronal differentiation, these switches of patterns are mainly regulated by changes in the expression of PTB (polypyrimidine tract binding) proteins, namely, PTBP1 and PTBP2, and SRRM4 (serine/arginine repetitive matrix protein 4) (Raj et al. 2015; Vuong et al. 2016), as illustrated in Fig. 3a.
Role of splicing factors during neurogenesis and neuron maturation. A PTBP1 is responsible for repressing the activation of neuronal genes and is highly expressed in neuronal stem cells and neuronal progenitor cells. Upon differentiation, PTBP1 becomes downregulated, allowing the induction of PTBP2 and PBX1 that will activate neuronal genes. SRRM4 also becomes expressed during neuronal differentiation and contributes to it by inactivating REST, a repressor of activation of neuronal genes. After the neurons become mature, the levels of PTBP2 decrease, giving rise to an adult neuronal splicing programme. NMD nonsense-mediated decay, NPC neural progenitor cell. B Once the neuronal cell fate commitment is achieved, neurons can migrate to generate the laminar structure of the brain. NOVA2 is a splicing factor particularly important for the cortical lamination since it regulates AS of Dab1 to promote neuronal migration. VZ ventricular zone, SVZ subventricular zone, IZ intermediate zone, CP cortical plate. C For the maturation process, neurons form synapses. This process is equally controlled by splicing factors, namely, KHDRBS2 that regulates neurexins (presynaptic cell-adhesion proteins), which are essential for synapse formation and transmission, and the NOVA family that regulates AS of neurotransmitter receptors
PTBP1 is expressed at high levels in neural stem cells and neural progenitor cells (NPC) but, upon neuronal differentiation, it becomes repressed, allowing the induction of PTBP2 expression, which in turn promotes NPC differentiation in postmitotic neurons (Li et al. 2014). In fact, it was shown that PTBP1 depletion in fibroblasts is enough to drive them towards a neuronal phenotype (Xue et al. 2013). Once the neurons become mature, the expression of PTBP2 is reduced, giving rise to an adult neuronal splicing programme (Linares et al. 2015). Together, the PTBP1 and PTBP2 interplay is thought to be responsible for 25% of neuron-specific AS events (Zaghlool et al. 2014) by coordinating splicing programmes through the use of a large set of target exons that display a range of responsiveness dependent on their levels of expression (Keppetipola et al. 2012; Linares et al. 2015). For instance, PTBP1 represses PBX1 (pre-B-cell leukaemia homeobox 1) exon 7, which creates an embryonic stem cell form of PBX1 that does not affect neuronal genes (Linares et al. 2015). Once PTBP1 is removed, the exon is included forming a PBX1 isoform able to promote the activation of neural genes. Another target of PTBP1 is PTBP2 exon 10, which is skipped when PTBP1 is expressed, producing a PTBP2 isoform that is degraded by nonsense-mediated decay (Spellman et al. 2007).
As mentioned above, the brain-specific SRRM4 also plays an important role during neurogenesis. Also known as nSR100, it targets several brain-specific exons in genes that are critical for nervous system development (Calarco et al. 2009). In fact, one of the most important roles of SRRM4 is the negative regulation of a transcriptional repressor of genes required for neurogenesis (REST). It promotes AS of REST transcripts to produce the REST4 isoform that has a reduced repressive activity, thus activating expression of REST targets in neural cells (Raj et al. 2011; Norris and Calarco 2012). Moreover, nSR100 directly outcompetes widespread neural exon repression by PTBP1 during early stages of neurogenesis (Raj et al. 2014).
Once the neuronal cell fate commitment is achieved, neurons and axons can migrate in a coordinated time and space manner to generate the laminar structure of the brain (Iijima et al. 2016). Two families of RBPs, NOVA (i.e. NOVA1 and NOVA2) and RBFOX (i.e. RBFOX1, RBFOX2, and RBFOX3), were linked to this neuronal development stage. NOVA1 is exclusively expressed in the subcortical regions and in postmitotic neurons of the central nervous system whereas NOVA2 is primarily expressed in the neocortex (Yano et al. 2010). In general, NOVA was shown to be important for the neuronal migration of mitotic progenitors and differentiated interneurons in the spinal cord as well as for axon outgrowth and guidance (Leggere et al. 2016). Moreover, NOVA2 was displayed as being important for cortical lamination since its absence causes the abnormal inclusion of the exon 7b and 7c in the DAB1 transcript, a component of the Reelin pathway that controls cortical neuronal migration and lamination (Yano et al. 2010; Norris and Calarco 2012) (see Fig. 3b).
Regarding the RBFOX family, RBFOX1 is expressed in neurons, heart and muscle and its absence in mouse, especially of isoform Rbfox1-iso2, causes defects during corticogenesis due to impairments in migration, axon growth and dendrite development of excitatory neurons (Hamada et al. 2015). RBFOX2, besides being expressed in all the aforementioned tissues, is also expressed in the embryo, hematopoietic stem cells and embryonic stem cells (ESCs) and plays a more critical role in the development of the cerebellum (Gehman et al. 2012). In fact, the absence of RBFOX2 affects the cerebellum by reducing its size and causing loss of foliation (Gehman et al. 2012). Moreover, RBFOX3 has been shown to be exclusively expressed in neurons and important for the promotion of neuronal differentiation of postmitotic neurons (Kim et al. 2013). Indeed, one of RBFOX3 targets is Numb, a crucial gene for the central nervous system (CNS) development since its loss of function in mice promotes deficiency in cranial neural tube closure and premature neuron production in the forebrain (Zhong et al. 2000; Kim et al. 2013). RBFOX1 is also responsible for the downregulation of RBFOX2 expression in RBFOX3-expressing cells (Dredge and Jensen 2011; Lin et al. 2016).
After the differentiation and migration processes are accomplished, neurons undergo a long period of formation and maturation of synapses. AS regulates crucial presynaptic cell-adhesion proteins for this stage named neurexins that are essential for synapse formation and transmission (Treutlein et al. 2014). This regulation is performed through the use of KHDRBS2 (KH-domain-containing, RNA-binding, signal-transduction-associated protein 2) (Iijima et al. 2014) (see Fig. 3c).
Other RBPs have also been shown to be important for synapse maturation. For instance, PTBP1 and PTBP2 are involved in synaptic maturation (Li et al. 2014) by regulating the expression of a scaffolding protein, PSD-95, that plays a key role during the synaptic maturation and plasticity of excitatory neurons (Zheng et al. 2012). NOVA seems to be equally relevant for the maturation of synapses as it regulates exons from genes that encode for neurotransmitter receptors or proteins that regulate the neurotransmitters release (Ule et al. 2005) (see Fig. 3c). ELAVL, MBNL, RBFOX1 and RBFOX3 are also reported as being important for the regulation of synaptic function (Wang et al. 2015c; Vuong et al. 2016; Lara-Pezzi et al. 2016). All this evidence supports the crucial role of AS in the different stages of the neuronal development in providing molecular tools necessary for the complex activity of the central nervous system.
Consistently with the described importance of AS in brain development and function, AS impairments are already known to be involved in several neurological diseases (Chabot and Shkreta 2016). Irimia and colleagues showed that most neuronal microexons (3–27 nucleotides) are misregulated in autism spectrum disorder and that this misregulation is linked to the downregulation of SRRM4 (Irimia et al. 2014). ELAVL2 was also shown to regulate transcripts related to autism (Berto et al. 2016). Mutations in RBFOX1 were likewise linked to autism, as well as with mental retardation and epilepsy (Mills and Michal 2012; Lee et al. 2016). Moreover, widespread alterations in splicing patterns of ion channel genes were linked to epilepsy and Alzheimer disease (Heinzen et al. 2007). In fact, AS was also shown to be playing a role in neurodegenerative disorders. Mutations in two RNA/DNA-binding proteins, TDP-43 and FUS/TLS, were found to be related with amyotrophic lateral sclerosis and frontotemporal lobar degeneration (Polymenidou et al. 2012; Cookson 2017). However, their role in these diseases seems to be complex and is not completely clear. Alzheimer’s disease-relevant genes, such as APP, TAU or APOE4, are known to undergo AS (Love et al. 2015) and shifts in the ratio of different types of SNCA isoforms are thought to play a role in Parkinson’s disease pathogenesis (La Cognata et al. 2015). Other splicing-related, not directly causative genes implicated in Parkinson’s disease, such as SRRM2, showed likewise condition-specific alterations in splicing regulation (La Cognata et al. 2015).
The frequent association of RNA regulatory dysfunction with neurological disorders demonstrate the relevance of AS in the nervous system (Nussbacher et al. 2015). However, its function as well as the mechanisms that underlie the regulation of splicing therein are still not fully elucidated. It is, therefore, necessary to expand our knowledge on those areas to improve therapies and diagnostic methods for neurological diseases.
Alternative splicing in gametogenesis
Spermatogenesis represents a continuous androgen-dependent developmental process defined by extensive transcriptional activity and reprogramming which is highly influenced by the interaction between germ and somatic cells. This unique regulatory mechanism guarantees faithful transition of spermatogonial stem cells throughout the meiosis process to produce haploid spermatocytes, as well as their subsequent differentiation into round spermatids and finally into functional spermatozoa.
In agreement with the notion that substantial modifications occur in the regulation of gene expression during this process, AS has been shown to be a predominant phenomenon in the testis. In fact, brain and testis are the anatomic sites where the highest levels of exon skip** events and the most specific expression of splicing-related genes take place (Yeo et al. 2004; Grosso et al. 2008; Barbosa-Morais et al. 2012). This testis-specific signature was observed in human, chimpanzee and mouse and includes the organ-specific expression of several splicing regulators such as SF3A2, SRPK1, SRPK2, as well as core snRNP components.
However, a considerable number of splicing events in human testis are not conserved in other closely related organisms and many of them account for non-functional protein products by introducing premature stop codons in transcripts’ sequences. Based on these observations, it has been proposed that part of the testis-specific splicing may represent “background” noise induced by high levels of cell proliferation, decrease of quality control or unspecific fluctuations in the expression of splicing regulators (Elliott and Grellscheid 2006). Nevertheless, several lines of evidence provide support for a relevant contribution of splicing regulation in spermatogenesis and fertility.
A classical example is the splicing-dependent reversal of the transcription factor CREM from a transcriptional repressor in premeiotic germ cells to a potent transcriptional activator in the pachytene spermatocyte stage (Foulkes et al. 1992). This functional switch regulates the expression of genes related to the differentiation of mature spermatozoa and, concordantly, infertile male patients with round spermatid maturation arrest express only the repressor version of CREM in the testis (Peri and Serio 2014). Further analyses have revealed that AS also plays critical roles during specific stages of sperm cell maturation, such as the biogenesis of the acrosome, an exocytotic vesicle present on the apical surface of the sperm head that is essential for the fusion with the oocyte plasma membrane. Acrosome formation is modulated by the two variants of proacrosin-binding protein ACRBP, the wild-type ACRBP-W and the intron 5-retaining splice variant ACRBP-V5, which are generated by AS of the Acrbp gene (Kanemori et al. 2013). A study in mouse epididymal sperm showed that ACRBP-V5 participates in the formation of the acrosomal granule into the centre of the acrosomal vesicle during early spermiogenesis, whereas ACRBP-W maintains proacrosin as an enzymatically inactive zymogen in the acrosome until acrosomal exocytosis in later stages (Kanemori et al. 2016). Moreover, it was recently shown that splice variants of the fibroblast growth factor receptors (FGFRs), known to regulate cell migration via PI3 K/Akt and MAPK/ERK signalling (Pintucci et al. 2002; Francavilla et al. 2013), are expressed in human testis and localise to the acrosomal region and the flagellum (Saucedo et al. 2015). Importantly, FGFRs shown activation in response to the FGF2 ligand, revealed by increased flagellar FGFR phosphorylation, which appeared associated with the activation of extracellular signal-regulated kinase ERK and Akt signalling pathways, as well as to increased sperm motility and sperm kinematics. It is therein hypothesised that FGF2, known to be present in the endometrium, the oviduct and in the oocyte vicinity (Malamitsi-Puchner et al. 2001), could bind to FGFR splice variants in the sperm acrosome to regulate fertilisation-related events.
A recent RNA-seq study evidenced a prominent reprogramming of the splicing environment during male meiosis in mice, identifying more than a hundred splicing switches, including skip** of exon 2 in the ODF2/Cenexin transcript, and mutually exclusive exons in the Ate1 gene (Schmid et al. 2013). ODF2 has been involved in a functional switch as microtubules organiser, moving from the centriole in somatic cells to the sperm tail in post-meiotic cells, whereas Ate1 encodes for a histone methyltransferase proposed to have important physiological roles in spermiogenic chromatin remodelling (Lambrot et al. 2012). Global changes in the levels of splicing regulators were also observed during spermatogenesis in this and other studies, including the upregulation of germ cell-specific Sam68, T-STAR, hnRNPGT, and RBMY proteins (Vernet and Artzt 1997; Venables et al. 2000, 2004; Paronetto et al. 2006) as well as alterations in the expression of non-germ cell-specific splicing factors, such as the downregulation of PTBP1, MBNL1, MBNL2, and hnRNPA1, and the upregulation of PTBP2/nPTB, BCAS2/SPF27, Tra2b, and the CUGBP ELAV-like proteins CELF1 (previously shown to be essential for normal spermatogenesis in mice) and CELF2 (Kress et al. 2007; Lambrot et al. 2012; Schmid et al. 2013; Liu et al. 2017).
The finding that members of the CELF protein group, including CELF1 and CELF2, were upregulated, while muscleblind proteins MBNL1 and MBNL2 appeared transcriptionally repressed during meiosis seems to be in agreement with the previously described antagonistic activity of CELF and muscleblind proteins (Kalsotra et al. 2008; Wang et al. 2015a; Solana et al. 2016). Moreover, the authors speculate that PTBP2 may functionally replace PTBP1 during meiosis, similar to what has been observed during neurogenesis (Boutz et al. 2007; Licatalosi et al. 2012), and suggested a suchlike replacement strategy of RBMX with RBMXL2/hnRNPGT. Consistently with these findings, an isoform-level expression profiling of genes located at the azoospermia factor (AZF) region at the Y chromosome identified 11 novel transcripts involved in human male infertility, including RBMX2, RBMXL1-1, and RBMXL1-2 (Ahmadi Rastegar et al. 2015). The same study proposed a diagnostic splicing-related signature that can be potentially used to effectively discriminate between premeiotic maturation arrest, Sertoli-cell-only syndrome, nonobstructive azoospermia, and normal testicular tissues, highlighting the importance of exploring spliced variants of candidate genes in spermatogenic failure.
RBM5 was also recently identified as a novel male germ cell splicing factor required for spermatid differentiation and male fertility (O’Bryan et al. 2013; Bao et al. 2014). A missense mutation in the second RNA recognition motif (RRM) of RBM5 appeared to induce shifts in its isoform ratios, as well as production of novelly spliced transcripts in putative RMB5 target genes, including members of the aforementioned MAPK/ERK signalling pathway (** events, suggesting that MRG15 may be a key regulator of splicing during spermiogenesis. To note, haploid spermatids experience a profound reorganisation and compaction of their chromatin, where a histone-based nucleosomal structure is extensively substituted by a protamine-based structure, a process that requires incorporation of testis-specific histone variants, post-translational histone modifications, chromatin-remodelling complexes, and transient formation of DNA breaks. Thus, the finding that AS may be coupled to histone dynamics during round spermatid stage leads to the proposal that regulation of pre-mRNA splicing by histone modifications can be an important conceptual element to understand spermatogenesis and epigenetic disorders in male infertile patients. In Fig. 4, a graphical representation of spermatogenesis and the associated AS program described in this section is depicted.
Graphical representation of spermatogenesis and its associated AS program. Temporal expression of key splicing factors and splice variants during meiotic division and spermatid maturation is represented by violet gradients. Bottom gradient panel shows the upregulation of the non-germ cell-specific splicing factors SPF27, RBM5, PTBP2, Tra2b, CELF1, and CELF2 and the germ cell-specific splicing factors (Sam68, T-STAR, hnRNPGT, and RBMY). Top gradient panel shows downregulation of the splicing factors PTBP1, MBNL1, MBNL2, and hnRNPA1. AS of the mRNA of the transcription factor CREM induces a functional switch from a transcriptional repressor in premeiotic cells to a transcriptional activator in the pachytene spermatocyte stage. Studies in mouse suggest that two splice variants of the proacrosin-binding protein ACRBP, ACRBP-V5, and ACRBP-W, participate in transport/packaging of proacrosin into acrosomal granules during spermiogenesis and in the promotion of acrosin release from the acrosome during acrosomal exocytosis, respectively. Similarly, splice variants of the fibroblast growth factor receptors (FGFRs) are expressed in spermatocytes and round spermatids and localise to the acrosomal region and the flagellum of mature sperm cells in humans
Finally, the role of AS in human infertility may not be restricted to the spermatogenesis process. The androgen receptor (AR), for instance, is a steroid receptor transcription factor playing important roles in human reproduction. Multiple AR AS variants have been involved in androgen insensitivity syndrome and associated male infertility (Dehm and Tindall 2011; Iwamori et al. 2016), but also in polycystic ovary syndrome, one of the most common causes of female infertility (Wang et al. 2015b). The mammalian follicle-stimulating hormone receptor (FSHR) gene encodes distinct splice variants resulting from exon skip** events that correlate with low response to ovarian stimulation with exogenous follicle-stimulating hormone (FSH) (Karakaya et al. 2014). These data suggest that alterations in the AS programme regulating hormone receptor pathways may be an important pathogenic mechanism in infertility. Further studies are required not only to validate in humans the abundant evidence for AS modulation observed during mouse spermatogenesis, but also to comprehensively characterise the global splicing regulatory mechanisms governing human germ cell differentiation and reproduction.
Alternative splicing in muscular tissues
The functional unit of myofibrils in striated muscular tissues is the sarcomere (Fig. 5a), a complex structure formed of overlap** protein filaments, whose dynamic sliding enables the shortening of the muscle fibre, ensuring contraction (Seeley et al. 2006; Squire 2016). Several studies reported that AS may play a fundamental role on the massive transcriptomic remodelling required during the transition from embryonic to adult muscle and for the dynamic functions required for contractile proteins in sarcomeres to achieve the demands of muscular tissues, such as contraction and force generation (Kalsotra et al. 2008; Giudice et al. 2014; Wang et al. 2016).
Alternative splicing of sarcomeric and membrane receptor proteins tunes muscular function. a Muscle contraction is achieved through the sliding between thin (rich in actin) and thick (rich in myosin) myofilaments of the sarcomere, shortening its length. Diversity of isoforms of sarcomeric proteins (such as titin, tropomyosin or troponin) required for tissue- or developmental stage-specific functions in muscular tissues arises by alternative splicing (sarcomere structure based on (Seeley et al. 2006)). b RNA-binding proteins MBNL1 and CELF1 are two major regulators of muscle-specific AS whose levels shift during the transition from embryonic to mature tissue. The calcium equilibrium needed for contraction of muscle cells is achieved by the coordinated activities of Ca2+ receptors at the membrane of the sarcoplasmic reticulum. Developmentally regulated AS of the sarcoplasmic/endoplasmic reticulum ATPase Ca2+ transporting (SERCA2) and ryanodine receptors (RyR) shapes calcium handling, controlling sarcomere contraction. Titin isoforms with different levels of stiffness change their relative abundance ratio in muscle cells during the transition from embryonic to adult tissue, altering myocardial compliance. The levels of the larger and more compliant titin isoform N2BA decrease with development, while the smaller and stiffer isoform N2B levels increase in mature and healthy muscle tissue. Troponin, one of the thin filament proteins, tunes the interactions between actin and myosin. MBNL1 and CELF1 regulate the inclusion of exon 5 of the cardiac troponin (cTNT) pre-mRNA by binding in the upstream or downstream intron, respectively. Tissue and developmental stage specificity of tropomyosin is achieved through the usage of alternative promoters and mutually exclusive exons of three of the four tropomyosin mammalian genes. In the case of the tropomyosin α gene, two alternative first exons and three sets of mutually exclusive exons contribute to the variability of tropomyosin isoforms
Muscle was one of the first tissues reported to have a specific pattern of AS (Llorian and Smith 2011). MBNL and CELF protein families have been consistently described as regulating muscle-specific AS events. MBNL1 typically modulates AS in muscle by repressing or promoting the inclusion of exons when binding to their upstream introns or downstream introns, respectively (Goers et al. 2010; Barash et al. 2010; Llorian and Smith 2011). The AS pattern during muscle development is regulated, among other factors, by an antagonism between the increased levels of MBNL1 and decreased expression of CELF1 (Pistoni et al. 2010) (see Fig. 5). In fact, a study with transgenic mice replicating the embryonic expression levels of CELF1 and MBNL1 in adult heart reproduces most of the embryonic splicing profile (Kalsotra et al. 2008). Other RBPs have been reported to regulate muscle-specific AS, such as RBFOX1 and polypyrimidine tract binding proteins, and other pairs of protein families with antagonistic functions in AS regulation have been established, such as CELF and PTB (Charlet et al. 2002; Sureau et al. 2011; Llorian and Smith 2011; Lara-Pezzi et al. 2013).
Most of the reported AS-associated alterations in muscular diseases are related to the loss of adult AS programmes and mimicking of the embryonic/develo** splicing profile, which is incompatible with function of developed tissues, namely, in the heart (Ho et al. 2004; Lee and Cooper 2009; Giudice et al. 2008; Giudice et al. 2009). In embryonic cardiac muscle, on the contrary, inclusion of exon 5 of cTNT is enhanced by the action of CELF2 in promoting and stabilising the binding of the spliceosomal component U2 snRNP, after binding to the downstream intron (Goo and Cooper 2009). Isoform diversity of tropomyosin (another thin filament protein) is expanded by the use of alternative promoters and mutually exclusive exons from the four tropomyosin genes (Tropomyosin α, β, γ, and δ), tuning actin/myosin interaction in sarcomeres in a developmental stage- and cell-specific manner (Gunning et al. 2005; Lara-Pezzi et al. 2013) (Fig. 5b).
The balance of Ca2+ inside the muscle fibre is controlled by a tight orchestration of membrane receptors’ function. One of the processes involved in muscle contraction is the release of Ca2+ from the sarcoplasmic reticulum through the ryanodine receptors (RyR). Two developmentally regulated alternatively spliced variants of the human cardiac RyR receptor (RYR2) have been reported to affect cardiomyocyte susceptibility to undergo apoptosis by differential regulation of nuclear and cytoplasmic Ca2+ release (George et al. 2007). The sarcoplasmic/endoplasmic reticulum ATPase Ca2+ transporting, SERCA2, is responsible for pum** Ca2+ back into the sarcoplasmic reticulum to achieve muscle relaxation. This calcium pump has been reported to have a cardiac and slow skeletal muscle-specific isoform, SERCA2a, and the switch to the ubiquitous isoform, SERCA2b, leads to impairment of the contractile function of the heart in mice (Ver Heyen et al. 2001) (Fig. 5b).
Titin is a giant sarcomeric protein responsible for the generation of passive tension by binding to myosin and myosin-binding protein C, enabling muscle flexibility and extensibility. Titin is known to undergo AS involving its 364 exons (Gigli et al. 2016; Zhu et al. 2016), and although a great number of titin isoforms can be generated, the adult cardiac muscle expresses two classes, whose ratios define the stiffness provided to the cardiomyocyte. The N2BA titin isoform is larger and contains additional spring elements that provide lower passive tension and more compliance to the cardiomyocyte, while the N2B isoform is smaller and stiffer, comprising 60–70% of adult human cardiac titin (Gigli et al. 2016). Moreover, AS of the titin gene has been linked to the regulatory activity of RNA binding motif protein 20 (RBM20), described as a regulator of cardiac AS and whose mutations have been associated with human dilated cardiomyopathy (Guo et al. 2012; Maatz et al. 2014; Zhu et al. 2016). Alterations in titin isoform balance were found during development of rat cardiac muscle, with N2BA levels decreasing and N2B levels increasing after birth (Opitz et al. 2004; Zhu et al. 2016). Also, a study on the expression of cardiac titin in patients with dilated cardiomyopathy reported alterations at the isoform ratio level favouring the more compliant N2BA isoform, with a consequent decrease in passive myocardial stiffness (Nagueh et al. 2004; Gigli et al. 2016) (Fig. 5b).
In both ends of the sarcomere, actin filaments are attached to a filamentous, proteic disc called the Z-line (Seeley et al. 2006). The LIM domain-binding protein 3 (LDB3) plays a role in muscle function by promoting sarcomere Z-line stability during contraction and its developmentally regulated isoforms are cardiac or skeletal muscle-specific (Cheng et al. 2011; Zhu et al. 2016). Also, a recent study focusing on splicing transitions from embryonic to adult muscle involved evaluating the effect of CELF1 re-expression in adult mouse cardiomyocytes and reported AS alteration in trafficking genes from adult to fetal patterns, resulting in multiple cardiac defects, namely, at the levels of T-tubule function, leading to impairment of the excitation–contraction coupling, calcium balance and force generation (Giudice et al. 2001; Mankodi et al. 2002; Lee and Cooper 2009; Pistoni et al. 2010).
Moreover, an RNA-seq study on postnatal AS transitions during heart development performed in mouse cardiomyocytes and cardiac fibroblasts reported that most alterations occurred before postnatal day 28, with an enrichment of AS transitions in genes related to vesicular trafficking and membrane alterations. This is consistent with the early life acquirement of an appropriate heart function, associated with proper membrane organisation, including correct ion channel functioning and ligand uptake, contributing to correct excitation/contraction coupling. Also, a substantial fraction of the AS events related to these transitions were enriched in binding motifs for CELF1, suggesting a direct mechanism for postnatal cardiac splicing regulation. To test the hypothesis of CELF1-regulated AS having a role in the assembly of the excitation–contraction apparatus, namely, in the invagination of the T-tubules, Giudice and colleagues induced re-expression of CELF1 in adult animals which was found to trigger important alterations in cardiac function in three different tests, with the T-tubule structure mimicking the one from postnatal days 10–15 (Giudice et al. 1997), and delivers inhibitory signals that counteract the co-stimulatory signal conferred by CD28 (Krummel and Allison 1995). Upon activation, CTLA4 expression is increased and exon 3, encoding a transmembrane domain, is included (Oaks et al. 2000), drastically increasing the expression of CTLA on the cell surface and empowering the T-cell inhibitory signal. TCR T-cell receptor, TMD transmembrane domain
In addition to the mentioned feedback mechanisms, immune activity is controlled by other mechanisms, such as apoptotic cell death, to avoid autoimmunity and assure T-cell homeostasis. The roles played by apoptosis include elimination of autoreactive T cells during maturation in the thymus and peripheral organs (central and peripheral T-cell tolerance), elimination of T cells activated for long in peripheral organs and also termination of the immune response (Abbas et al. 2014). Several genes involved in apoptosis are alternatively spliced, such as FAS, which encodes a death receptor. Skip** of its exon 6, containing the transmembrane domain, leads to the production of a soluble protein; inclusion of that exon results in a membrane receptor that can trigger signalling pathways leading to cell death (Hughes and Crispe 1995). This exon skip** event is regulated by TIA-1 and TIAR hnRNPs in a feed-forward mechanism (Izquierdo et al. 2005; Izquierdo and Valcárcel 2007). The importance of apoptotic regulation in the immune system is highlighted by the fact that higher levels of the soluble, anti-apoptotic FAS isoform are detected in patients with systemic lupus erythematosus (SLE) and mice injected with this isoform display autoimmune diseases (Cheng et al. 1994).
Deregulation of splicing events that are necessary for normal function of the immune system often leads to a wide range of diseases, from which we highlight autoimmune diseases. These usually result from defective self-tolerance or regulation, due to impaired deletion of autoreactive lymphocytes, or low numbers of cells that regulate the immune response, such as regulatory T cells (Abbas et al. 2014).
Several SNPs affecting genes with relevant roles in the immune system have been described as leading to aberrant splicing patterns. An SNP in exon 4 of the aforesaid PTPRC gene, encoding for CD45, leads to the inclusion of that exon by disturbing an exonic splicing silencer. While the isoform lacking variable exons is expressed in activated T cells, to regulate activity, this polymorphism resulting in increased expression of the longer isoform is one of those linked to multiple sclerosis (MS) (Lynch and Weiss 2001; Evsyukova et al. 2010). Several other genes involved in immune system function have SNPs linked to autoimmune diseases. For instance, an SNP affecting a branch point site in the BANK1 gene, encoding for a protein involved in B-cell receptor signalling, induces skip** of the constitutive exon 2 and has been linked to SLE (Kozyrev et al. 2008). Other reports have linked skip** of exon 9 of protein-tyrosine phosphatase sigma (PTPRS) to ulcerative colitis (Muise et al. 2007) and reduced splicing efficiency of intron 1 of inositol 1,4,5-trisphosphate 3-kinase C (ITPKC) to Kawasaki disease (Onouchi et al. 2008).
Interestingly, AS has also been proposed to generate epitopes to which the organism has not been tolerized. Central tolerance only covers a set of isoforms but, in autoimmunity-prone conditions, expression of the remaining, non-tolerized isoforms increases, which may trigger an immune response (Ng et al. 2004). One example is the myelin proteolipid protein (PLP), present in either a longer isoform or a shorter one, lacking exon 3B. PLP is expressed in the thymus, but this expression is restricted to the short isoform, so the host does not acquire tolerance to exon 3B. In pathogenic conditions, damage is exerted to myelin and the longer isoform is exposed, which may trigger an autoimmune reaction that contributes to MS (Klein et al. 2000).
Alternative splicing has been shown to provide an extra layer of regulation in the immune system, from the reprogramming of B and T cells upon activation to the generation of the diversity that characterises this complex and dynamic system. Disruption of this regulatory layer by SNPs can lead to diseases, particularly to autoimmunity, which underscores the importance of unveiling new mechanisms and alterations to splicing regulation in the context of the immune system.