Abstract
Existing data on Myxozoa parasites infecting mullets were reviewed. The validity of nine species names was updated. Sixteen species were registered during analysis of original material collected in the Mediterranean, Black, Azov, and Japan Seas in 2004–2005. A new bivalvulid myxozoan parasite, Myxobolus adeli n. sp., was described from the inner organs of the golden grey mullet Liza aurata (Risso, 1810) collected in the Mediterranean (Ebro Delta, Spain), Black Sea (Kerch Strait, Ukraine), and Azov Sea (Genichesk, Ukraine) coastal waters. It is characterized by the presence of elongated, spindle-like cysts 0.5–1.3 mm in size, filled with wide transverse-oval spores about 6.2 × 7.2 × 4.6 μm in size, with two equal polar capsules measuring about 3.0 × 1.8 μm and short polar filament, turned into four coils. The obtained data show that this species differs from all previously described Myxobolus spp. with equal polar capsules. Comparative study of Myxobolus spp. recorded in worldwide mullets indicates a close relationship with M. adeli n. sp. and Myxobolus improvisus Isjumova, 1964 registered in mullets. Probably, the last species includes representatives of some different species, infecting freshwater and marine hosts.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The mullets (Mugiliformes: Mugilidae) have a worldwide distribution and inhabit tropical and temperate waters (Nelson 1984). According to current data (FishBase) the Mugilidae family includes 24 genera and 72 species, inhabiting tropical, subtropical, and the southern part of the Atlantic, Indian, and Pacific oceans. Many mullet species have comparatively trivial areas, but one of them—grey mullet Mugil cephalus (Linnaeus, 1758)—can be cosmopolitan, spreading including the coastal waters of Europe, Asia, Africa, Australia, America, and Oceania. Mullets have been used as a considerable source of food in different parts of the world. The importance of mullet for aquaculture and the pathologic potential of some parasites, in particular Myxosporea, motivate their detailed study. Myxosporea represents one of the important groups of parasites infecting worldwide mullets (Lom and Dyková 1992; Kent et al. 2001). So far, a few revisionary studies of parasites infecting worldwide mullets have been conducted by Paperna (1975). Twelve species of Myxozoa were reviewed by Paperna and Overstreet (1981). The genera Sphaerospora, Henneguya, Myxidium, Myxosoma, Myxobolus, Kudoa, infecting mullets, were revisionary studied by Sitjà-Bobadilla and Alvarez-Pellitero (1994), Jajasri and Hoffman (1982), Landsberg and Lom (1991), Eiras 2002, Eiras et al. (2005), and Moran et al. (1999).
In the last decades, geography of the mullet parasites studies and knowledge about myxosporeans infecting worldwide mullets were considerably widened. The aim of this paper is to investigate the biodiversity of myxozoans based on existing data and original material obtained during parasitological investigations of mullets in the Mediterranean, Black, Azov, and Japan Seas. Studies were supported by INTAS project (INTAS Ref. No.: 03-51-5998).
Material and methods
The original study was carried out on data obtained during parasitological investigations of 3,362 fish specimens. Mullets were caught in May–June and October–November 2004–2005. In the Mediterranean coastal region of Spain (Ebro River Estuary and Santa Pola Bay) 1,550 specimens of mullets belonging to five genera were dissected. In the Ponto-Azov region, Ukraine (coastal waters near Kerch, Genichesk, Berdiansk, and Mariupol), 1,498 mullets representing four genera were dissected. Material from the Japan Sea was presented by results of parasitological dissections of 314 mullets from two genera caught in the Russian coastal regions of Japan Sea (Razdolnaya River, Kievka Bay, Posiete Bay, Artemovka). Parasitological analysis was performed based on partial parasitological dissection (Bykhovskaya-Pavlovskaya 1985). Fresh spores were fixed on slides in a glycerine jelly medium according to Donets and Schulman (1973). Spores were photographed and measured on digital images. Descriptions of the spores were based on the references of Schulman et al. (1997) and Lom and Arthur (2006). Live and Giemsa-stained spores were observed and measured under MBI-3 and Olympus BX50F4 microscope equipped with Analysis Pro 2.11 software.
For ultrastructural analyses, infected tissues were fixed in a 2.5 % (v/v) glutaraldehyde in 0.1 M sodium cacodylate buffer (pH 7.4) for several days at 4 °C. After washing twice with 0.1 M sodium cacodylate buffer and post-fixation in 2.0 % (v/v) osmium tetroxide in cacodylate buffer for 1 h at 4 °C, the pieces were dehydrated and embedded in Epon–Araldite solution using a standard procedure (Vávra and Maddox 1976). Blocks of embedded tissues were sectioned with an LKB III ultra-microtome. Semi-thin sections were stained with methylene blue. Ultrathin sections were mounted on copper grids, double-stained with uranyl acetate and lead citrate, and examined in a JEM 100B electron microscope operated at 80 kV.
Results and discussion
Myxosporeans of the worldwide mullets
By the present time, 64 myxosporean species from 13 genera and nine families infecting 16 mullet species belonging to six genera have been registered (Table 1). Five species were identified to the genus range. The majority of myxosporeans parasitizing mullets are attributed to the family Myxobolidae. Among them, 32 and two species belong to the genera Myxobolus and Henneguya, correspondingly. Eleven species belong to the family Myxidiidae, eight representatives of Zschokkella genus, and three species belong to the genus Myxidium. Ten species were found as representatives of the family Kudoidae belonging to a single genus Kudoa. The family Sphaerosporidae contains four species belonging to the genus Sphaerospora. One species from Alataspora and one from Pseudalataspora genera were registered as representatives of the Alatasporidae family. Sphaeromyxidae, Ortholineidae, Chloromyxidae, Polysporoplasmidae as well as the Sinuolineidae family are represented by single species of each genus (Sphaeromyxum, Ortholinea, Chloromyxum, Polysporoplasma).
The maximum of species richness of Myxosporea was registered in flathead mullet M. cephalus. Thirty six species of myxosporeans from eight genera were mentioned in named host. The area includes the Mediterranean basin, Red Sea, Atlantic Coast of Africa, Mexican Gulf, and Indian and Pacific Ocean coastal waters.
Golden grey mullet Liza aurata (Risso, 1810) was mentioned as the host of 18 species of Myxosporea infecting different organs of the host in the Mediterranean, Black, and Azov Seas. Lea** mullet Liza saliens (Risso, 1810) is a host of nine species of Myxozoa, found in the Black, Azov, Mediterranean, Adriatic, and Caspian Seas. Nine species of myxosporeans were also found in thinlip mullet Liza ramada (Risso, 1810) from the Mediterranean basin. Six species of Myxosporea were described in thicklip grey mullet Chelon labrosus (Risso, 1827) and in redlip mullet Liza haematocheila Temminck & Schlegel, 1845 in the Japan Sea (Russia), in Liaohe River (China), and in Black and Azov Seas (Ukraina). From the Indian shores, three species of myxosporeans were found in largescale mullet Liza macrolepis (Smith, 1846) and two species in corsula Rhinomugil corsula (Hamilton, 1822) and in yellowtail mullet Sicamugil cascasia (Hamilton, 1822). One species was described from squaretail mullet Liza vaigiensis (Quoy & Gaimard, 1825), Liza parsia (Hamilton, 1822), and longarm mullet Valamugil cunnesius (Valenciennes, 1836), from Mugil japonica and keeled mullet Liza carinata (Valenciennes, 1836), white mullet Mugil curema Valenciennes, 1836 and Mugil platanus.
Among the species of myxosporeans, described from mullets, 17 species were found in the gall bladder. In the gills, muscles, and kidney, consequently, six, five, and four species of myxosporeans were registered. Three myxozoans species were found in the mesenterium and intestines; two in the heart, on fins, and scales. The urinary bladder, spleen, and liver were infected with a separate species of myxozoans. Eighteen species were detected in various organs (Table 1).
There are only six cosmopolite species. All of them are parasites of M. cephalus. Those are Myxobolus muelleri, Myxobolus ichkeulensis, Myxobolus episquamalis, Myxobolus exiguus, Myxobolus parvus, and Myxobolus spinacurvatura.
Original data of the author’s investigations
We conducted taxonomical studies of mullet myxosporeans collected in the Mediterranean, Black, Azov, and Japan Seas in the summer and autumn 2004–2005. M. cephalus was parasitologically studied in all regions; L. haematocheila—in the Japan, Black, and Azov Seas; L. aurata and L. saliens—in the Mediterranean, Black, and Azov Seas; and L. ramada and C. labrosus—exclusively in the Mediterranean Sea.
Totally, 16 species of myxosporeans have been registered. New information about myxosporean fauna for each region of investigations has been received.
Zschokkella admiranda from M. cephalus has been registered for the first time in the Mediterranean fauna. Sphaeromyxa sabrazesi, Kudoa unicapsula, Alataspora sp., Z. admiranda, Myxobolus adeli sp. n., M. parvus, M. muelleri, M. ichkeulensis, M. spinacurvatura, Myxobolus rohdei, M. exiguus, Myxobolus nile, Myxobolus episquamalus have been found in the coastal waters of Spain. M. cephalus appeared to be a new host for S. sabrazesi. L. aurata was registered as a new host for Sphaerospora dicentrarchi. L. ramada and C. labrosus were found as hosts for Polysporoplasma mugilis in the Mediterranean Sea. P. mugilis infecting L. aurata has been found for the first time in the Black Sea. S. dicentrarchi, M. ichkeulensis, and M. spinacurvatura infecting M. cephalus was firstly registered in the Black and Azov Seas. L. aurata was firstly registered as a new host for Z. admiranda. M. ichkeulensis, M. spinacurvatura, and M. episquamalus parasitizing M. cephalus has been found for the first time in the Japan Sea.
Among mullets inhabiting the Mediterranean basin, we found several myxosporeans, already known species of parasites, which were described earlier as new species. All of them were synonymized. Species names Sphaerospora mugili Yurakhno & Maltsev, 2002; Sphaerospora sp. Quaglio et al., 2002; and Sphaerospora sp. Caffara et al., 2003 were considered as younger synonyms of S. dicentrarchi Sitja-Bobadilla & Alvarez-Pellitero, 1992. Others species names containing synonyms are presented by as follows: Myxobolus bizerti Bahri & Marques, 1996 (=Myxobolus hannensis Fall et al., 1997); Myxobolus ichkeulensis Bahri & Marques, 1996 (=Myxobolus goreensis Fall et al., 1997), M. adeli sp. n. (=Myxobolus improvisus Isjumova, 1964 (in Schulman 1966; Yurakhno and Maltsev 2002); Myxobolus lizauratus (in Yurakhno and Ovcharenko 2008).
In the present paper, we describe the following new species: M. adeli sp. n. from L. aurata in the Mediterranean, Black, and Azov Seas.
Myxobolus adeli sp. nov. (Table 2; Figs. 2, 3, 28)
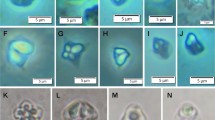
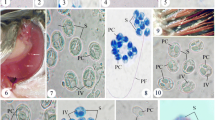
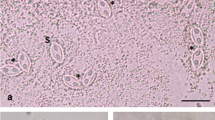
Light microscope and ultrastructural data of some myxozoan parasitizing collected mullets. 1 Spores of M. muelleri. 2, 3 M. adeli sp. nov., spores (2) and spindle-shaped cysts of different maturity (3). 4, 7 M. episquamalis. Compact whitish masses on the distal parts of scales (4). Each cystic mass consists of numerous microcysts. Oval spores tapered at the anterior end (7). Polar capsules equal and pyriform. 5, 6 Spherical spores of M. ichkeulensis with oval polar capsules. No intercapsular appendix is visible (6). 8–13 M. parvus. Spores (8–11) and rounded-to-oval white cysts up to 2.0 mm in diameter (12, 13). Polar capsules contain four coils of longitudinally twisted polar filament (10). Two valvogenic cells form a good developed sutural ring (11). 14 Spores of M. nile with unequal polar capsules. 15 Spores of M. spinacurvatura. Polar capsules do not reach the midpoint of the spore length. 16–17 Alataspora sp. Spherical polar capsules located close to the anterior pole (16). Vegetative stages presented by rounded or oval-shaped bisporous plasmodia with transparent ectoplasm and small-grained endoplasm (17). 18 Kudoa trifolia. Four small subspherical polar capsules are located in the central part of the spore, between the spore body and leaf-like appendages. 19 M. rohdei. Spores are regularly ellipsoidal with a good developed sutural edge around the spore, bearing distinct sutural markings. 20 Z. admiranda. Round or oval disporous plasmodia with small granular endoplasm. Oval spores with rounded poles. 21 Spores of S. dicentrarchi. 22 P. mugilis. Spores subspherical in front view. Sutural line straight. Polar capsules spherical, of equal size. 23 S. sabrazesi. Spores cylindrical, bent in arch form; with truncated ends. Polar capsules large, cylindrical. 24–27 Light and electron microscope data of the spores of K. unicapsula. K. unicapsula and K. trifolia—mix infection (25). 26–27 Ultrastructure of the spores of K. unicapsula (26, 27). Transverse (26) and cross (27) sections through the basal part of the spore showing unequal polar capsules and four shelves. Big polar capsule contains two coils of polar filament. 28—Spore construction of M. adeli sp. nov. Host infected: M. cephalus (4–7, 14, 15, 19, 21, 23); L. aurata (1, 8–13, 20, 22, 24–27); L. ramada (2, 3, 16–18, 28). Sites: intestine (1, 8–13, 15, 19, 24–27), pyloric caeca (2, 3), scales (4, 7), gills (14), gall bladder (16, 18, 20, 21, 23), and kidney (22)
Type host. Golden mullet L. aurata (Risso, 1810)
Site of infection. Intestine, pyloric caeca, esophagus, stomach, swim bladder; sporadically: gills and muscles
Locality. Mediterranean coastal waters (Ebro River Delta, Santa Pola Bay), Black Sea waters (Kerch Channel), and Azov Sea (Genichesk aquatoria)
Prevalence. Ebro River Delta, Spain, autumn 2005—11 % (8/73); Santa-Pola Bay, Spain, summer 2005—12 % (7/60); Kerch Channel, Ukraine, summer 2004—13 % (11/83), autumn 2005—11 % (4/35); Genichesk, Ukraine, summer 2004—6 % (11/188), autumn 2005—9 % (4/47)
Description. Vegetative forms: cysts are spindle form with sharpened or rounded ends, 0.5–1.3 mm in size. Spores: oval shaped, transversally widened. Widely positioned pyriform polar capsules close acquired at the anterior pole and occupy half or a more than a half of the spore cavity. Polar capsules of equal sizes. Suture line well expressed; sometimes slightly folded. Spore dimensions from glycerine jelly mounts were 6.19 ± 0.29 μm (5.56–6.75) in length; 7.22 ± 0.28 μm (6.57–7.77) in width, and 4.60 ± 0.36 μm (3.55–5.27) in thickness (n = 50). Polar capsules measured 3.07 ± 0.32 μm (2.36–3.8) × 1.81 ± 0.22 μm (1.26–2.28). Four coiled polar filament measured 13.45 ± 1.95 μm (12.0–17.76) in length.
Syntype specimens. Glass slides numbers AAK 7, 15, 19, 20, 21, 22, 23, 29, 33, 37, 44; AAG 6, 8, 13, 38, 42, 51, 63, 64, 136, 147, 148; MAE 31; 2 MAE 21, 26, 39, 56, 65; 2 MAS 3, 4, 5, 6, 7, 8, 11, 12; 3 MAE 17, 20, 49; 3 MAS 4, 7, 8, 13, 17, 32, 35; and 4 MAE 9, 10, 12, 18, 23, 24, 29, 31 were deposited in the collection of the Department of Parasitology of Institute of Biology of the Southern Seas of National Academy of Sciences of Ukraine, 2 Nakhimov Avenue, 99011, Sevastopol, Ukraine
Etymology. Species is called to the honor of Adel Kovalyova, expert on Myxosporea studies, who worked longtime in the Institute of Biology of the Southern Seas (IBSS) and Fish Diseases Laboratory AtlantNIRO, Kaliningrad, Russia
Taxonomic summary. The new myxosoporean species differs from other representatives of the genus Myxobolus by morphology and spore sizes. The spore shape and/or measurements of the present species showed some similarities with Myxosporea from the Eurasia freshwater hosts: M. improvisus Isjumova, 1964 in. Schulman 1966; Myxobolus latus Schulman, 1962 and Myxobolus artus Achmerov, 1960. M. adeli sp. produces spindle-shaped plasmodia contrary to M. improvisus and M. latus with round- or oval-shaped (M. artus) vegetative stages. The spores of newly described species are comparatively smaller than the spores of all three related species. M. adeli sp. n. differs from M. improvisus also by equal sized polar capsules (Table 2).
Alataspora sp. (Table 3; Figs. 16, 17)
Type host. Thinlip mullet L. ramada (Risso, 1826)
Site of infection. Gall bladder
Locality. Mediterranean coastal waters (Ebro River Delta, Santa Pola)
Prevalence. 2.7 % (1/37) in 2004; 0.9 % (1/109) in 2005
Description. Vegetative stages presented by rounded or oval-shaped bisporous plasmodia with transparent ectoplasm and small-grained endoplasm. Spores are strongly elongated in the plane perpendicular to the sutural line. They have clearly expressed triangular part, cavity of which contains polar capsules and amoeboid germ. Elongated top parts of the valves form single wing-like appendages slightly unequal in sizes. Suture line is straight and clear. Spherical polar capsules are located close to the anterior pole and open near the suture line to one side of spore. Amoeboid germ is located under polar capsules.
Spore measurements presented in Table 3.
Taxonomic summary. Based on the spore construction, Alataspora sp. occupies intermediate position between representatives of Alataspora and Pseudalataspora genera. It resembles Alataspora solomoni Yurakhno, 1988, differing from it by unequal length of valves and larger spores and polar capsules. We consider Alataspora sp. a species inquirenda that needs a precise species description after obtaining of additional data.
References
Asejeva NL (1994) Detection on Myxosoma acutum in pilengas from Japan Sea. Izvestiya TINRO 117:157–158 (In Russian)
Asejeva NL (2000) Myxosporeans of anadrome and marine coastal fishes of north-west part of Japan Sea. Izvestiya TINRO 127:593–606 (In Russian)
Bahri S, Marques A (1996) Myxosporean parasites of the genus Myxobolus from Mugil cephalus in Ichkeul lagoon, Tunisia: description of two new species. Dis Aquat Org 27(2):115–122
Bahri S, Andree KB, Hedrick RP (2003) Morphological and phylogenetic studies of marine Myxobolus spp. from mullet in Ichkeul Lake, Tunisia. J Eukaryot Microbiol 50(6):463–470
Bykhovskaya-Pavlovskaya IE (1985) Parasites of fish: guide for the study. Nauka, Leningrad, 123 p; (In Russian)
Chen CL, Hsieh SR (1984) New species of Myxidium (Myxosporidia) from freshwater fishes of China. In: Parasitic organisms of freshwater fish of China. Institute of Hydrobiology, Academia Sinica, Agricultural Publishing House, Bei**g pp 89–8 (in Chinese with English abstract)
Diamanka A, Fall M, Diebakate C, Faye N, Toguebaye BS (2008) Identification of Myxobolus episquamalis (Myxozoa, Myxobolidae) in flathead mullet Mugil cephalus (Pisces, Teleostei, Mugilidae) from the coast of Senegal (eastern tropical Atlantic Ocean). Acta Adriat 49(1):19–23
Diamant A, Ucko M, Paperna I, Colorni A, Lipshitz A (2005) Kudoa iwatai (Myxosporea: Multivalvulida) in wild and cultured fish in the Red Sea: redescription and molecular phylogeny. J Parasitol 91:1175–1189
Domnich IF, Sarabeev VL (1999) Parasitic fauna of Azov Sea grey mullet and the ways of its formation. Visnyk Zaporizkogo Derzhavnogo Universytetu (Vistnyk Zaporizhzhya National Univ) 2:218–223 (In Ukrainian)
Domnich IF, Sarabeev VL (2000) The present fauna of fish parasites in the northern part of Azov Sea. Visnyk Zaporizkogo Derzhavnogo Universytetu (Vistnyk Zaporizhzhya National Univ) 1:224–230 (In Ukrainian)
Donets ZS (1979) The zoogeographical analysis of Myxosporidia in the USSR southern water reservoirs. In: Evolution and ecology of Sporozoa and Cnidosporidia. Tr Zool Inst Akad Nauk SSSR 87:65–90 (In Russian)
Donets ZS, Schulman SS (1973) About methods of Myxosporidia (Protozoa, Cnidosporidia) investigation. Parazitologiya 7(2):191–193
Dorothy KP, Kalavati C (1992) Two new myxosporean parasites of the mullet Liza macrolepis (Smith). Uttar Pradesh J Zool 12(1):15–19
Egusa S, Maeno Y, Sorimachi M (1990) A new species of Myxozoa, Myxobolus episquamalis sp. n. infecting the scales of the mullet, Mugil cephalus L. Fish Pathol 25(2):87–91
Eiras JC (2002) Synopsis of the species of the genus Henneguya Thelohan, 1892 (Myxozoa: Myxosporea: Myxobolidae). Syst Parasitol 52:43–54
Eiras JC, D’Souza J (2004) Myxobolus goensis n. sp. (Myxozoa, Myxosporea, Myxobolidae), a parasite of the gills of Mugil cephalus (Osteichthyes, Mugilidae) from Goa, India. Parasite 11:243–248
Eiras JC, Molnar K, Lu YS (2005) Synopsis of the species of Myxobolus Butschli, 1882 (Myxozoa: Myxosporea: Myxobolidae). Syst Parasitol 61:1–46
Eiras JC, Abreu PC, Robaldo R, Pereira Junior J (2007) Myxobolus platanus n. sp. (Myxosporea, Myxobolidae), a parasite of Mugil platanus Gunther, 1880 (Osteichthyes, Mugilidae) from Lagoa dos Patos, RS, Brasil. Arq Bras Med Vet Zootec 59(4):895–898
Ergens R, Gussev AV, Izyumova NA, Molnar K (1975) Parasite fauna of fishes of the Tisa River Basin. Praha 117 p
Fall M, Kpatcha KP, Diebakate C, Faye N, Toguebaye BS (1997) Observations sur des Myxosporidies (Myxozoa) du genre Myxobolus parasites de Mugil cephalus (Poisson, Téléostéen) du Sénégal. Parasite 2:173–180
Faye N, Kpatcha K, Fall M, Toguebaye BS (1997) Heart infections due to myxosporean (Myxozoa) parasites in marine and estuarine fishes from Senegal. Bull Eur Assoc Fish Pathol 17(3/4):115–117
Fujita T (1930) On the new Myxosporidia Chloromyxum bora nov. sp. In the muscles of the Gray-Mullet. Dibitsugaku zasshi Tokyo 42:45–48
Haldar DP, Samal KK, Mukhopadhyaya D (1996) Studies on the protozoan parasites of fishes in Orissa: eight species of Myxobolus Butschli (Myxozoa: Bivalvulida). J Bengal Nat History Soc 16:3–24
Holzer AS, Blasco-Costa I, Sarabeev VL, Ovcharenko MO, Balbuena JA (2006) Kudoa trifolia sp. n.—molecular phylogeny suggests a new spore morphology and unusual tissue location for a well-known genus. J Fish Dis 29:743–755
Ibragimov SR (1987) Forming of the parasites fauna of mullets in the Caspian Sea. Baku, Institute of Zoology of AS Aserbaijan SSR (Deposited by VINITI 03.04.87, № 2407-B87):1–14 (In Russian)
Iskov MP (1989) Myxosporidia (Myxosporea). In: Markevitch AP, Schulman SS (eds) Fauna Ukrainy, vol. 37 (4), Naukowa Dumka, Kiev: 212 p. (In Russian)
Iversen ES, Chitty N, van Meter N (1971) Some myxosporida from marine fishes in south Florida. J Protozool 18(1):82–86
Jajasri M, Hoffman GL (1982) Review of Myxidium (Protozoa: Myxozoa: Myxosporea). Protozool Abstr 6:61–91
Kalavati C, Anuradha I (1993) Two new species of myxosporeans infecting Valamugil cunnesius in Visakhaptnam harbour, east coast of India. Uttar Pradesh J Zool 13:148–152
Kalavati C, Anuradha I (1995) A new myxosporean, Bipteria indica sp. n. (Myxospora: Sinuolineidae) from the gall bladder of the striped mullet, Mugil cephalus L. Acta Protozool 34(4):307–309
Karatajev AK, Iskov MP (1984) The materials on the fauna of protozoa—fish parasites in the Black Sea north-western part. Vestn Zool 6:13–16 (In Russian)
Kent ML, Andree KB, Bartholomew JL, El-Matbouli M, Desser SS, Delvin RH, Feist SW, Hedrick RP, Hoffmann RW, Khattra J, Hallett SL, Lester RJG, Longshaw M, Palenzuela O, Siddall ME, **zo CX (2001) Recent advances in our knowledge of the Myxozoa. J Eukaryot Microbiol 48:395–413
Kim WS, Kim JH, Jang MS, Jang SJ, Oh MJ (2013) Infection of wild mullet (Mugil cephalus) with Myxobolus episquamalis in Korea. Parasitol Res 112(1):447–451
Kolesnikova MG, Donets ZS (1987) The fauna of fish Myxosporidia at the Crimean coast. IV-th All Union Symposium “Parasitology and Pathology of marine organisms” (21–23 April, 1987, Kaliningrad): Abstracts:89–90 (In Russian)
Kovaleva AA, Donets ZS, Kolesnikova MG (1989) New species of Myxosporidia (Cnidospora, Myxosporea) in the Black Sea fish. Vestn Zool 5:75–79 (In Russian)
Kpatcha TK, Faye N, Diebakate C, Fall M, Toguebaye BS (1997) Nouvelles espèces d.Henneguya Thelohan, 1895 (Myxozoa, Myxosporea) parasites des poissons marins du Sénégal: étude en microscopie photonique et électronique. Ann Sci Nat Zool Paris 18:81–91
Kudo R (1919) A synopsis on genera and species of Myxosporidia. Illinois Biol Monogr 5(3–4):1–265
Landsberg JH, Lom J (1991) Taxonomy of the genera of the Myxosoma/Myxobolus group (Myxobolidae: Myxosporea), current listing of species and revision of synonyms. Syst Parasitol 18:165–186
Lom J, Arthur JR (2006) A guideline for the preparation of species descriptions in Myxosporea. J Fish Dis 12(2):151–156
Lom J, Dyková I (1992) Protozoan parasites of fishes. Developments in aquaculture and fisheries sciences. Elsevier, Amsterdam, 314 pp
Lom J, Dyková I (1994) Studies on protozoan parasites of Australian fishes III. Species of the genus Myxobolus Bűtschli, 1882. Eur J Protistol 30(4):431–439
Lubat V, Radujkovic B, Marques A, Bouix G (1989) Parasites des poissons marins du Montenegro: Myxosporidies. Acta Adriat 30(1–2):31–50
Maeno Y, Sorimachi M, Ogawa K, Egusa S (1990) Myxobolus spinacurvatura sp. n. (Myxosporea: Bivalvulida) parasitic in deformed mullet, Mugil cephalus. Fish Pathol 25(1):37–41 (In Japanese)
Maeno Y, Nagasawa K, Sorimachi M (1993) Kudoa intestinalis n. sp. (Myxosporea: Multivalvulida) from the intestinal musculature of the striped mullet, Mugil cephalus, from Japan. J Parasitol 79(2):190–192
Moran JDW, Whitaker DJ, Kent ML (1999) A review of the myxosporean genus Kudoa Meglitsch, 1947, and its impact on the international aquaculture industry and commercial fisheries. Aquaculture 172:163–196
Munoz P, Palenzuela O, Alvarez-Pelitero P, Sitja-Bobadilla A (1999) Comparative studies on carbohydrates of several myxosporean parasites of fish using lectin histochemical methods. Folia Parasitol 46:241–247
Naidenova NN, Schulman SS, Donets ZS (1975) Type protozoa, class Myxosporidia. In: Guide of parasites of vertebrates of Black and Azov Seas Naukova Dumka Kiev:20–50 (In Russian)
Narasimhamurti CC (1970) Myxosoma intestinalis n. sp. (Protozoa, Myxosporidia) parasiting in the intestinal epithelium in the estuaria fish Mugil waigensis. Proc Indian Acad Sci 71(1):19–27
Narasimhamurti CC, Kalavati C (1979a) Myxosoma lairdi n. sp. (Protozoa: Myxosporidia) parasitic in the gut of the estuarine fish Liza macrolepis Smith. Proc Indian Acad Sci 88:269–272
Narasimhamurti CC, Kalavati C (1979b) Kudoa tetraspora n. sp. (Protozoa: Myxosporidea: Protozoa) parasitic in the brain tissue of Mugil cephalus. Proc Indian Acad Sci 88:85–89
Narasimhamurti С, Kalavati С, Saratchandra B (1980) Myxosoma microspora n. sp. (Myxosporidia: Protozoa) parasitic in the gills of Mugil cephalus. J Fish Biol 16:345–348
Negm-Eldim NM, Govedich FR, Davies RW (1999) Gill myxosporeans on some Egyptian freshwater fish. Deutsche Tierarzt Wochenschr 106:459–465
Nelson JS (1984) Fishes of the world, 2nd edn. Wiley, New York, 523 p
Özak AA, Demirkale I, Cengizler I (2012) Two new records of Myxobolus Butschli, 1882 (Myxozoa, Myxosporea, Myxobolidae) species from Turkey. Turk J Zool 36(2):191–199
Padros F, Palenzuela O, Hispano C, Tosas O, Zarza C, Crespo S, Alvarez-Pellitero P (2001) Myxidium leei (Myxozoa) infections in aquarium-reared Mediterranean fish species. Dis Aquat Org 47(1):57–62
Paperna I (1975) Parasites and disease of the grey mullet (Mugilidae) with special reference to the seas of the Near East. Aquaculture 5:65–80
Paperna I, Overstreet RM (1981) Parasites and diseases of mullets (Mugilidae). In: Oren OH (ed) Aquaculture of grey mullets. IBP 26. Cambridge University Press, UK
Parenzan P (1966) Myxobolus mugilis e Myxobolus branchialis nuovi missosporidi parassiti di Mugil chelo dello Jonio. Bull Soc Nat Napoli 73:3–8
Parisi B (1912) Primo contributo alla distribuzione geographica dei Missosporidi in Italia (Milano). Atti Soc Ital Sci Nat 50:283–290
Pedro-Andrĕs MB, Marques A, Gracia-Royo MP (2011) Myxosporean infection of grey mullet in the Ebro Delta: identification and ultrastructure of Myxobolus ichkeulensis Bahri & Marques, 1996 infecting the gills of Mugil cephalus L. Acta Protozool 50:67–71
Perugia A (1891) Sulle Missosporidie dei Pesci marini. I. Boll Sci 12:34–139
Pogoreltceva TP (1952) The materials on the fish parasite fauna of the Black Sea southern-eastern part. Tr Inst Zool Akad Nauk Ukr SSR 8:100–120
Pogoreltceva TP (1964) Materials on investigations of parasitic Protozoa of Black Sea fishes. Problemy Parazitologii. Tr Ukr Resp Nauchn O-va Parazitol 3:16–29
Pulsford A, Matthews RA (1982) An ultrastructural study on Myxobolus exiguus Thelohan, 1895 (Myxosporea) from grey mullet Crenimugil labrosus (Risso). J Fish Dis 5(6):509–526
Quaglio F, Delgado ML, Caffara M, Florio D, Marcer F, Fioravanti ML, Restani R (2002) Histopathological observations in marine farmed fish infected by Myxosporidia. II Osservazioni istopatologiche in pesci marini d’allevamento affetti da mixosporidiosi. II. Boll Soc Ital Patol Ittica 14(34):44–67
Reshetnikova AV (1955) Parasitic fauna of the Black sea mullets. Tr Karadag Biol Stn Akad Nauk Ukr SSR 13:71–95 (In Russian)
Rothwell JT, Virgona JL, Callinan RB, Nicholls PJ, Langdon JS (1997) Occurrence of cutaneous infections of Myxobolus episquamalis (Myxozoa: Myxobolidae) in sea mullet, Mugil cephalus L. in Australia. Aust Vet J 75(5):349–355
Sarabeev VL, Domnich IF (2000) The age dynamics in haarder Mugil soiuy infection in the sea of Azov Molochny gulf. Vestn Zool Supp 14(2):6–12 (In Russian)
Sarkar NK (1989) Myxobolus anili sp. nov. (Myxozoa: Myxosporea) from a marine teleost fish Rhinomugil corsula Hamilton. Proc Zool Soc Calcutta 42(1–2):71–74
Sarkar NK (1999) Some new Myxosporidia (Myxozoa: Myxosporea) of the genera Myxobolus Butschli, 1882 Unicapsula Davis, 1942 Kudoa Meglitsch, 1947 Ortholinea Shulman, 1962 and Neoparvicapsula Gajevskaya, Kovaleva and Shulman, 1982. Proc Zool Soc Calcutta 52(1):38–48
Sarkar NK, Chaudhury SR (1996) Kudoa cascasia sp. n. (Myxosporea: Kudoidae) parasitic in the mesentery of Sicamugil cascasia (Ham.) from Hoodghly estuary of West Bengal, India. Acta Protozool 35:335–338
Sarkar NK, Ghosh S (1991) Two new coelozoic myxosporida (Myxozoa: Myxosporea) from estuarine teleost fishes (Mugilidae) of West Bengal, India. Proc Zool Soc Calcutta 44(2):131–135
Schulman SS (1957) About pathogenicity of myxosporea Myxobolus exiguus and it-associated epizooties. Izv Vses Nauchno-Issled Inst Ozern Rechn Rybn Khoz 42:328–329 (In Russian)
Schulman SS (1962) Class Cnidosporidia. In: Opredelitel parasitov presnovodnykh ryb SSSR AN SSSR, Moscow :47–130 (In Russian)
Schulman SS (1966) Myxosporidia of the fauna of the USSR. Nauka, Moscow, 504 p. (In Russian)
Schulman SS, Donets ZS, Kovaleva AA (1997) Class of myxosporeans (Myxosporea) of the world fauna. Vol 1. Nauka, St-Petersburg, 567p
Siau Y (1978) Contribution á la coinnaissance des Microsporidies: etude de Myxobolus exiguous Thélohan, 1895 (cytology, cycle, actions sur l’hôte, epidemiologie). Thesis USTL (Université Sciences et Techniques du Lanquedoc), Montpellier II
Sitjà-Bobadilla A, Alvarez-Pellitero P (1993) Zschokkella mugilis n. sp. (Myxosporea: Bivalvulida) from mullets (Teleostei: Mugilidae) of Mediterranean waters: light and electron microscopic description. J Eukaryot Microbiol 40(6):755–764
Sitjà-Bobadilla A, Alvarez-Pellitero P (1994) Revised classification and key species of the genus Sphaerospora Davies, 1917 (Protozoa: Myxosporea). Res Rev Parasitol 54(2):67–80
Sitjà-Bobadilla A, Alvarez-Pellitero P (1995) Light and electron microscopic description of Polysporoplasma n. g. (Myxosporea: Bivalvulida), Polysporoplasma sparis n. sp. from Sparus aurata (L.), and Polysporoplasma mugilis n. sp. from Liza aurata L. Eur J Protistol 31:77–89
Sitjà-Bobadilla A, Alvarez-Pellitero P (1996) Virus-like particles in Polysporoplasma mugilis (Protozoa: Myxosporea), parasitic in a marine fish (Liza aurata L.). Int J Parasitol 26(4):457–459
Syirovatka NI, Nizova GA (2000) Formation of haarder parasitic fauna in the Azov basin water reservoirs. In: Trudy AzNIIRH (1998–1999). The main problems of fish economics and protection of fish farms water reservoirs in the Azov-Black sea basin, (Ed. Makarov), BKI, Rostov-on-Don: 172–176 (In Russian)
Thélohan P (1895) Recherches sur les Myxosporidies. Bull Sci Fr Belg 26:100–394
U-Taynapun K, Penprapai N, Bangrak P, Mekata T, Itami T, Tantikitti C (2011) Myxobolus supamattayai n. sp. (Myxosporea: Myxobolidae) from Thailand parasitizing the scale pellicle of wild mullet (Valamugil seheli). Parasitol Res 109(1):81–91
Vávra J, Maddox JV (1976) Methods in microsporidiology. In: Bulla LA, Cheng TC (eds) Comparative pathobiology. Plenum Press, New York, pp 281–319
Yemmen C, Ktari MH, Bahri S (2012) Parasitofauna of some mugilid and soleid fish species from Tunisian lagoons. Acta Adriat 52(1):173–182
Yurakhno VM (1993) New data of the fauna of myxosporidians from fishes of the Black Sea. Parazitologiya 27(4):320–326 (In Russian)
Yurakhno VM (2004) The fauna of myxosporeans (Protozoa: Myxosporea) of fishes in the Black Sea and its seasonal and interannual aspects of variability. In: Nigmatullin CM (ed) Sovremennyje problemy parazitologii, zoologii i ekologii. KGTU, Kaliningrad, pp 160–171 (In Russian)
Yurakhno VM, Maltsev VN (2002) New data on myxosporeans of mullets in the Atlantic Ocean basin. Ekologija Morya 61:39–42 (In Russian)
Yurakhno VM, Ovcharenko M (2008) Myxosporeans of the world ocean mullets. Proceedings of the IV Congress of the Russian Society of Parasitologists – Russian Academy of Sciences, held 20–25 October 2008 at the Zoological Institute RAS, St. Petersburg, “Parasitology in XXI century – problems, methods, solutions”, Vol. 3, St. Petersburg: 231–234
Yurakhno VM, Ovcharenko MO, Holzer AS, Sarabeev VL, Balbuena JA (2007) Kudoa unicapsula n. sp. (Myxosporea: Kudoidae) a parasite of the Mediterranean mullets Liza ramada and L. aurata (Teleostei: Mugilidae). Parasitol Res 101(6):1671–1680
Acknowledgments
The authors are much indebted to Dr. Volodimir Sarabeev and Dr. Nataliya Rubtsova, Biology Faculty, of Zaporizhzya National University, Ukraine; Dr. Viacheslav Maltsev, Zonal Specialiżed State Laboratory of Veterinary Medicine, Kerch, Ukraine; and Dr. Liudmila Shvetsova, Section of Hydrobiont Diseases, Pacific Research Fisheries Centre, Vladivostok, Russian Federation for the help in material collection and field research work. We are also grateful to Dr. Juan Antonio Balbuena, University of Valencia, Spain for coordination of research in a board of INTAS project tasks. The research was supported by the INTAS grant no. 03-51-5998.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution License which permits any use, distribution, and reproduction in any medium, provided the original author(s) and the source are credited.
About this article
Cite this article
Yurakhno, V.M., Ovcharenko, M.O. Study of Myxosporea (Myxozoa), infecting worldwide mullets with description of a new species. Parasitol Res 113, 3661–3674 (2014). https://doi.org/10.1007/s00436-014-4031-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00436-014-4031-5