Abstract
Purpose
Sphingomyelin (SM) hydrolysis by sphingomyelinase (SMase) has become an important signalling pathway, with the product ceramide implicated in regulation of cell growth, differentiation and apoptosis. Alkaline SMase is specifically located in the intestinal tract. Marked reductions of the enzyme activity have been found in sporadic colorectal carcinomas and in both adenomas and flat mucosa of patients with familial adenomatous polyposis, indicating an anti-proliferative role in colonic cell growth.
Methods
We examined the effects of a purified alkaline SMase from rat intestine and a bacterial neutral SMase on cell growth parameters in HT-29 colonic carcinoma cells.
Results
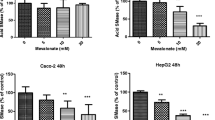
Alkaline SMase was found to inhibit proliferation of HT-29 cells in both dose-dependent and time-dependent manners. The threshold concentration of the enzyme was approximately 2.5 μU/ml, and the maximum effect was obtained at approximately 20 μU/ml, which inhibited the cell growth by 50%. The inhibition occurred rapidly, and maximum effect was reached after 12 h of incubation. Dose-dependent inhibition of DNA synthesis was also demonstrated. The effect of alkaline SMase was preceded and accompanied by increased hydrolysis of SM and production of ceramide. Neutral SMase with equivalent hydrolytic capacity did not inhibit cell growth. Alkaline SMase did not induce apoptosis in HT-29 cells. Alkaline SMase did not inhibit growth of IEC-6 cells.
Conclusion
Alkaline SMase, at doses that induce SM hydrolysis, inhibits growth of colon cancer cells. The inhibition is attributed to an anti-proliferative effect rather than an apoptotic effect.






Similar content being viewed by others
References
Andrieu N, Salvayre R, Levade T (1996) Comparative study of the metabolic pools of sphingomyelin and phosphatidylcholine sensitive to tumor necrosis factor. Eur J Biochem 236:738–745
Bligh EH, Dyer WJ (1959) A rapid method for total lipid extraction and purification. Can J Biochem Physiol 37:911–918
Chan TA, Morin PJ, Vogelstein B, Kinzler KW (1998) Mechanisms underlying nonsteroidal antiinflammatory drug-mediated apoptosis. Proc Natl Acad Sci U S A 95:681–686
Chatterjee S (1993) Neutral sphingomyelinase. Adv Lipid Res 26:25–48
Chatterjee S, Han H, Rollins S, Cleveland T (1999) Molecular cloning, characterization, and expression of a novel human neutral sphingomyelinase. J Biol Chem 274:37407–37412
Cheng Y, Tauschel H-T, Nilsson Å, Duan R-D (1999) Administration of ursodeoxycholic acid increases the activities of alkaline sphingomyelinase and caspase-3 in rat colon. Scand J Gastroenterol 34:915–920
Dillehay DL, Webb SK, Schmelz E-M, Merrill AH (1994) Dietary sphingomyelin inhibits 1,2-dimethylhydrazine-induced colon cancer in CF1 mice. J Nutr 124:615–620
Duan RD, Nilsson Å (1999) Enzymes hydrolysing sphingolipids in gastrointestinal tract. Methods Enzymol 311:276–286
Duan R-D, Nyberg L, Nilsson Å (1995) Alkaline sphingomyelinase activity in rat gastrointestinal tract: distribution and characterization. Biochim Biophys Acta 1259:49–55
Duan R-D, Hertervig E, Nyberg L, Hauge T, Sternby B, Lillienau J, Farooqi A, Nilsson Å (1996) Distribution of alkaline sphingomyelinase activity in human beings and animals. Dig Dis Sci 41:1801–1806
Dudeja PK, Dahiya R, Brasitus TA (1986) The role of sphingomyelin and sphingomyelinase in 1,2-dimethyhydrazine-induced lipid alterations of rat colonic plasma membranes. Biochim Biophys Acta 863:309–312
Earnest DL, Holubec H, Wali RK, Jolley CS, Bissonette M, Bhattacharyya AK, Roy H, Khare S, Brasitus TA (1994) Chemoprevention of azoxymethane-induced colonic carcinogenesis by supplemental dietary ursodeoxycholic acid. Cancer Res 54:5071–5074
Elder DJ, Paraskeva C (1997) NSAIDs to prevent colorectal cancer: a question of sensitivity. Gastroenterology 113:1999–2003
Hannun YA, Linardic CM (1993) Sphingolipid breakdown products: anti-proliferative and tumor-suppressor lipids. Biochim Biophys Acta 1154:223–236
Hannun YA, Obeid LM (1995) Ceramide as intracellular signal for apoptosis. TIBS 20:73–77
Hedlund M, Duan RD, Nilsson A, Svanborg C (1998) Sphingomyelin, glycosphingolipids and ceramide signalling in cells exposed to P-fimbriated Escherichia coli. Mol Microbiol 29:1297–1306
Hertervig E, Nilsson A, Nyberg L, Duan RD (1997) Alkaline sphingomyelinase activity is decreased in human colorectal carcinoma. Cancer 79:448–453
Hertervig E, Nilsson Å, Björk J, Hultkrantz R, Duan R-D (1999) Familial adenomatous polyposis is associated with a marked decrease in alkaline sphingomyelinase activity; a key factor to the unrestrained cell proliferation. Br J Cancer 81:232–236
Hertervig E, Nilsson Å, Nilbert M, Duan R-D (2003) Reduction in alkaline sphingomyelinase in colorectal tumorigenesis is not related to the APC gene mutations. Int J Colorectal Dis 18:309–313
Kolesnick RN (1991) Sphingomyelin and derivatives as cellular signals. Prog Lipid Res 30:1–38
Levade T, Jaffrézou J-P (1999) Signalling sphingomyelinases: which, where, how and why? Biochim Biophys Acta 1438:1–17
Linardic CM, Hannun YA (1994) Identification of a distinct pool of sphingomyelin involved in the sphingomyelin cycle. J Biol Chem 269:23530–23537
Merchant TE, Diamantis PM, Lauwers G, Haida T, Kasimos JN, Guillem J, Glonek T, Minsky BD (1995) Characterization of malignant colon tumors with 31P nuclear magnetic resonance phospholipid and phosphatic metabolite profiles. Cancer 76 1715–1723
Nilsson Å (1969) The presence of sphingomyelin- and ceramide-cleaving enzymes in the small intestinal tract. Biochim Biophys Acta 176:339–347
Nyberg L, Duan R-D, Axelsson J, Nilsson Å (1996) Identification of an alkaline sphingomyelinase activity in human bile. Biochim Biophys Acta 1300:42–48
Nyberg L, Nilsson Å, Lundgren P, Duan R-D (1997) Localization and capacity of sphingomyelin digestion in the rat intestinal tract. J Nutr Biochem 8:112–118
Okazaki T, Bielawska A, Bell RM, Hannun YA (1990) Role of ceramide as a lipid mediator of 1,25 dihydroxyvitamine D3-induced HL-60 cell differentiation. J Biol Chem 265:15823–15831
Schmelz EM, Dillehay DL, Webb SK, Reiter A, Adams J: Merrill AH Jr (1996) Sphingomyelin consumption suppresses aberrant colonic crypt foci and increases the proportion of adenomas versus adenocarcinomas in CF1 mice treated with 1,2-dimethylhydrazine: implications for dietary sphingolipids and colon carcinogenesis. Cancer Res 56:4936–4941
Schmelz EM, Dombrink-Kurtzman MA, Roberts PC, Kozutsumi Y, Kawasaki T, Merrill AH Jr (1998). Induction of apoptosis by fumonisin B1 in HT29 cells is mediated by the accumulation of endogenous free sphingoid bases. Toxicol Appl Pharmacol 148:252–260
Spence MW (1993) Sphingomyelinases. Adv Lipid Res 26:3–23
Stoffel W (1975) Chemical synthesis of choline-labeled lecithins and sphingomyelins. Methods Enzymol 35:533–541
Tomiuk S, Hofmann K, Nix M, Zumbansen M, Stoffel W (1998) Cloned mammalian neutral sphingomyelinase: functions in sphingolipid signaling? Proc Natl Acad Sci U S A 95:3638–3643
Veldman RJ, Klappe K, Hoekstra D, Kok JW (1998) Metabolism and apoptotic properties of elevated ceramide in HT29rev cells. Biochem J 331:563–569
Acknowledgements
This work was supported by grants from the Swedish Medical Council (12156 and 03969), the Swedish Cancer foundation (000307), the Albert Påhlsson Foundation, the Crafoord Foundation, the Swedish Society of Medicine, the Institution of Medicine of the University Hospital, and the Medical Faculty of Lund University.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hertervig, E., Nilsson, Å., Cheng, Y. et al. Purified intestinal alkaline sphingomyelinase inhibits proliferation without inducing apoptosis in HT-29 colon carcinoma cells. J Cancer Res Clin Oncol 129, 577–582 (2003). https://doi.org/10.1007/s00432-003-0466-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00432-003-0466-2