Abstract
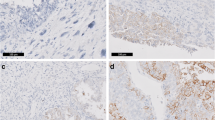
Several subtypes of high-grade endometrial carcinomas (ECs) contain an undifferentiated component of non-epithelial morphology, including undifferentiated and dedifferentiated carcinomas and carcinosarcomas (CSs). The mechanism by which an EC undergoes dedifferentiation has been the subject of much debate. The epithelial–mesenchymal transition (EMT) is one of the mechanisms implicated in the transdifferentiation of high-grade carcinomas. To improve our understanding of the role of EMT in these tumors, we studied a series of 89 carcinomas including 14 undifferentiated/dedifferentiated endometrial carcinomas (UECs/DECs), 49 CSs (21 endometrial, 29 tubo-ovarian and peritoneal), 17 endometrioid carcinomas (grade 1–3), and 9 high-grade serous carcinomas of the uterus, using a panel of antibodies targeting known epithelial markers (Pan-Keratin AE1/AE3 and E-cadherin), mesenchymal markers (N-cadherin), EMT transcription factors (TFs) (ZEB1, ZEB2, TWIST1), PAX8, estrogen receptors (ER), progesterone receptors (PR), and the p53 protein. At least one of the three EMT markers (more frequently ZEB1) was positive in the sarcomatous component of 98% (n = 48/49) of CSs and 98% (n = 13/14) of the undifferentiated component of UEC/DEC. In addition, 86% of sarcomatous areas of CSs and 79% of the undifferentiated component of UEC/DEC expressed all three EMT-TFs. The expression of these markers was associated with the loss of or reduction in epithelial markers (Pan-keratin, E-cadherin), PAX8, and hormone receptors. In contrast, none of the endometrioid and serous endometrial carcinomas expressed ZEB1, while 6% and 36% of endometrioid and 11% and 25% of serous carcinomas focally expressed ZEB2 and TWIST1, respectively. Although morphologically different, EMT appears to be implicated in the dedifferentiation in both CSs and UEC/DEC. Indeed, we speculate that the occurrence of EMT in a well differentiated endometrioid carcinoma may consecutively lead to a dedifferentiated and undifferentiated carcinoma, while in a type II carcinoma, it may result in a CS.


Similar content being viewed by others
References
WHO Classification of tumours of female reproductive organs. Fourth Edition—WHO—OMS. [En ligne]. Disponible sur: http://apps.who.int/bookorders/anglais/detart1.jsp?codlan=1&codcol=70&codcch=4006. Consulté le: 25-Juill-2017
Thiery JP (2002) Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer 2(6):442–454
Romero-Pérez L et al (2013) ZEB1 overexpression associated with E-cadherin and microRNA-200 downregulation is characteristic of undifferentiated endometrial carcinoma. Mod Pathol Off J U S Can Acad Pathol Inc 26(11):1514–1524
Zeisberg M, Neilson EG (2009) Biomarkers for epithelial-mesenchymal transitions. J Clin Invest 119(6):1429–1437
Thiery J-P (2009) Epithelial-mesenchymal transitions in cancer onset and progression. Bull Acad Natl Med 193(9):1969–1978; discussion 1978–1979
Puisieux A, Brabletz T, Caramel J (2014) Oncogenic roles of EMT-inducing transcription factors. Nat Cell Biol 16(6):488–494
Nieto MA, Huang RY-J, Jackson RA, Thiery JP (2016) EMT: 2016. Cell 166(1):21–45
Iwano M, Plieth D, Danoff TM, Xue C, Okada H, Neilson EG (2002) Evidence that fibroblasts derive from epithelium during tissue fibrosis. J Clin Invest 110(3):341–350
Brabletz T et al (2001) Variable β-catenin expression in colorectal cancers indicates tumor progression driven by the tumor environment. Proc Natl Acad Sci U S A 98(18):10356–10361
Brabletz T (2012) To differentiate or not—routes towards metastasis. Nat Rev Cancer 12(6):425–436
Peinado H, Olmeda D, Cano A (2007) Snail, Zeb and bHLH factors in tumour progression: an alliance against the epithelial phenotype? Nat Rev Cancer 7(6):415–428
Hugo H et al (2007) Epithelial—mesenchymal and mesenchymal—epithelial transitions in carcinoma progression. J. Cell. Physiol. 213(2):374–383
Caramel J et al (2013) A switch in the expression of embryonic EMT-inducers drives the development of malignant melanoma. Cancer Cell 24(4):466–480
Ansieau S et al (2008) Induction of EMT by twist proteins as a collateral effect of tumor-promoting inactivation of premature senescence. Cancer Cell 14(1):79–89
Valsesia-Wittmann S et al (2004) Oncogenic cooperation between H-Twist and N-Myc overrides failsafe programs in cancer cells. Cancer Cell 6(6):625–630
Morel A-P et al (2012) EMT inducers catalyze malignant transformation of mammary epithelial cells and drive tumorigenesis towards claudin-low tumors in transgenic mice. PLoS Genet 8(5):e1002723
Mani SA et al (2008) The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 133(4):704–715
Morel A-P, Lièvre M, Thomas C, Hinkal G, Ansieau S, Puisieux A (2008) Generation of breast cancer stem cells through epithelial-mesenchymal transition. PLoS ONE 3(8):e2888
Morel A-P et al (2017) A stemness-related ZEB1–MSRB3 axis governs cellular pliancy and breast cancer genome stability. Nat Med 23(5):568–578
Colas E et al (2012) The EMT signaling pathways in endometrial carcinoma. Clin Transl Oncol Off Publ Fed Span Oncol Soc Natl Cancer Inst Mex 14(10):715–720
Montserrat N et al (2012) Epithelial to mesenchymal transition in early stage endometrioid endometrial carcinoma. Hum Pathol 43(5):632–643
Dong P, Konno Y, Watari H, Hosaka M, Noguchi M, Sakuragi N (2014) The impact of microRNA-mediated PI3K/AKT signaling on epithelial-mesenchymal transition and cancer stemness in endometrial cancer. J Transl Med 12:231
Köbel M, Ronnett BM, Singh N, Soslow RA, Gilks CB, McCluggage WG (2019) Interpretation of P53 immunohistochemistry in endometrial carcinomas: toward increased reproducibility. Int J Gynecol Pathol Off J Int Soc Gynecol Pathol 38(Suppl 1):S123–S131
Recommendations for the immunohistochemistry of the hormonal receptors on paraffin sections in breast cancer. Update 1999 (1999) Group for Evaluation of Prognostic Factors using Immunohistochemistry in Breast Cancer (GEFPICS-FNCLCC). Ann Pathol 19(4):336–343
Friedl P, Gilmour D (2009) Collective cell migration in morphogenesis, regeneration and cancer. Nat Rev Mol Cell Biol 10(7):445–457
Thiery JP, Acloque H, Huang RYJ, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139(5):871–890
Ye X, Weinberg RA (2015) Epithelial-mesenchymal plasticity: a central regulator of cancer progression. Trends Cell Biol 25(11):675–686
Stewart CJR, McCluggage WG (2013) Epithelial-mesenchymal transition in carcinomas of the female genital tract. Histopathology 62(1):31–43
Ribatti D (2017) Epithelial-mesenchymal transition in morphogenesis, cancer progression and angiogenesis. Exp Cell Res 353(1):1–5
Gregory PA et al (2008) The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol 10(5):593–601
Park S-M, Gaur AB, Lengyel E, Peter ME (2008) The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev 22(7):894–907
Burk U et al (2008) A reciprocal repression between ZEB1 and members of the miR-200 family promotes EMT and invasion in cancer cells. EMBO Rep 9(6):582–589
Bracken CP et al (2008) A double-negative feedback loop between ZEB1-SIP1 and the microRNA-200 family regulates epithelial-mesenchymal transition. Cancer Res 68(19):7846–7854
Stewart CJR, Crook ML (2015) Fascin expression in undifferentiated and dedifferentiated endometrial carcinoma. Hum Pathol 46(10):1514–1520
**ng P et al (2011) Fascin, an actin-bundling protein, promotes breast cancer progression in vitro. Cell Biochem. Funct. 29(4):303–310
Mao X, Duan X, Jiang B (2016) Fascin induces epithelial-mesenchymal transition of cholangiocarcinoma cells by regulating Wnt/β-catenin signaling. Med Sci Monit Int Med J Exp Clin Res 22:3479–3485
Hayashi Y, Osanai M, Lee G-H (2011) Fascin-1 expression correlates with repression of E-cadherin expression in hepatocellular carcinoma cells and augments their invasiveness in combination with matrix metalloproteinases. Cancer Sci 102(6):1228–1235
Richmond AM, Blake EA, Torkko K, Smith EE, Spillman MA, Post MD (2017) Fascin is associated with aggressive behavior and poor outcome in uterine carcinosarcoma. Int J Gynecol Cancer 27:1895–1903
Ramalingam P, Masand RP, Euscher ED, Malpica A (2016) Undifferentiated carcinoma of the endometrium: an expanded immunohistochemical analysis including PAX-8 and basal-like carcinoma surrogate markers. Int J Gynecol Pathol Off J Int Soc Gynecol Pathol 35(5):410–418
Onder S et al (2017) High expression of SALL4 and fascin, and loss of E-cadherin expression in undifferentiated/dedifferentiated carcinomas of the endometrium: an immunohistochemical and clinicopathologic study. Medicine (Baltimore) 96(10):e6248
Kuhn E, Ayhan A, Bahadirli-Talbott A, Zhao C, Shih I-M (2014) Molecular characterization of undifferentiated carcinoma associated with endometrioid carcinoma. Am J Surg Pathol 38(5):660–665
Rosa-Rosa JM et al (2016) Molecular genetic heterogeneity in undifferentiated endometrial carcinomas. Mod Pathol Off J U S Can Acad Pathol Inc 29(11):1390–1398
Tessier-Cloutier B, Soslow RA, Stewart CJR, Köbel M, Lee C-H (2018) Frequent loss of claudin-4 expression in dedifferentiated and undifferentiated endometrial carcinomas. Histopathology 73(2):299–305
Lin X, Shang X, Manorek G, Howell SB (2013) Regulation of the epithelial-mesenchymal transition by claudin-3 and claudin-4. PloS One 8(6):e67496
Sánchez-Tilló E et al (2010) ZEB1 represses E-cadherin and induces an EMT by recruiting the SWI/SNF chromatin-remodeling protein BRG1. Oncogene 29(24):3490–3500
Karnezis AN et al (2016) Loss of switch/sucrose non-fermenting complex protein expression is associated with dedifferentiation in endometrial carcinomas. Mod Pathol Off J U S Can Acad Pathol Inc 29(3):302–314
Romero-Pérez L et al (2013) Molecular events in endometrial carcinosarcomas and the role of high mobility group AT-hook 2 in endometrial carcinogenesis. Hum Pathol 44(2):244–254
Castilla MÁ et al (2011) Micro-RNA signature of the epithelial-mesenchymal transition in endometrial carcinosarcoma. J Pathol 223(1):72–80
Chiyoda T et al (2012) Expression profiles of carcinosarcoma of the uterine corpus-are these similar to carcinoma or sarcoma? Genes. Chromosomes Cancer 51(3):229–239
Saegusa M, Hashimura M, Kuwata T, Okayasu I (2009) Requirement of the Akt/beta-catenin pathway for uterine carcinosarcoma genesis, modulating E-cadherin expression through the transactivation of slug. Am J Pathol 174(6):2107–2115
Díaz-Martín J et al (2014) A core microRNA signature associated with inducers of the epithelial-to-mesenchymal transition. J Pathol 232(3):319–329
Pang A, Carbini M, Moreira AL, Maki RG (2018) Carcinosarcomas and related cancers: tumors caught in the act of epithelial-mesenchymal transition. J Clin Oncol Off J Am Soc Clin Oncol 36(2):210–216
Cherniack AD et al (2017) Integrated molecular characterization of uterine carcinosarcoma. Cancer Cell 31(3):411–423
Zhao S et al (2016) Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc Natl Acad Sci U S A 113(43):12238–12243
de Jong RA, Nijman HW, Wijbrandi TF, Reyners AK, Boezen HM, Hollema H (2011) Molecular markers and clinical behavior of uterine carcinosarcomas: focus on the epithelial tumor component. Mod Pathol Off J U S Can Acad Pathol Inc 24(10):1368–1379
Tanaka Y et al (2013) Prognostic impact of EMT (epithelial-mesenchymal-transition)-related protein expression in endometrial cancer. Cancer Biol Ther 14(1):13–19
Kim T et al (2011) p53 regulates epithelial-mesenchymal transition through microRNAs targeting ZEB1 and ZEB2. J Exp Med 208(5):875–883
Chang C-J et al (2011) p53 regulates epithelial-mesenchymal transition and stem cell properties through modulating miRNAs. Nat Cell Biol 13(3):317–323
Dong P et al (2013) Mutant p53 gain-of-function induces epithelial-mesenchymal transition through modulation of the miR-130b-ZEB1 axis. Oncogene 32(27):3286–3295
Ansieau S, Courtois-Cox S, Morel A-P, Puisieux A (2011) Failsafe program escape and EMT: a deleterious partnership. Semin Cancer Biol 21(6):392–396
Author information
Authors and Affiliations
Contributions
Tatiana Franceschi: writing of the article and immunohistochemical analyses.
Emeline Durieux: immunohistochemical analyses and selection of the cases and of the paraffin blocks.
Anne Pierre Morel: choosing and providing antibodies for the study and critical review of the manuscript.
Pierre de Saint Hilaire: selection of the patients and surgery and providing surgical tumor material for the study.
Isabelle Ray-Coquard: selection of the patients,
Alain Puisieux: designing the study, critical review of the article.
Mojgan Devouassoux-Shisheboran: designing the study and writing of the article.
Corresponding author
Ethics declarations
The study was approved by the Ethics Committee of the Medical Board (CHU Lyon). This study was conducted in accordance with the Declaration of Helsinki.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Franceschi, T., Durieux, E., Morel, A.P. et al. Role of epithelial–mesenchymal transition factors in the histogenesis of uterine carcinomas. Virchows Arch 475, 85–94 (2019). https://doi.org/10.1007/s00428-019-02532-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00428-019-02532-w