Abstract
Main conclusion
Molecular and microscopic analyses reveal enormous non-cultivable endophytic bacteria in grapevine field shoots with functional significance. Diverse bacteria enter tissue cultures through surface-sterilized tissues and survive surreptitiously with varying taxonomic realignments.
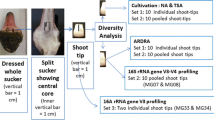
The study was envisaged to assess the extent of endophytic bacterial association with field shoot tissues of grapevine and the likelihood of introduction of such internally colonizing bacteria in vitro adopting molecular techniques targeting the non-cultivable bacterial community. PowerFood®-kit derived DNA from surface-sterilized field shoot tips of grapevine Flame Seedless was employed in a preliminary bacterial class-specific PCR screening proving positive for major prokaryotic taxa including Archaea. Taxonomic and functional diversity were analyzed through whole metagenome profiling (WMG) which revealed predominantly phylum Actinobacteria, Proteobacteria, and minor shares of Firmicutes, Bacteroidetes, and Deinococcus-Thermus with varying functional roles ascribable to the whole bacterial community. Field shoot tip tissues and callus derived from stem segments were further employed in 16S rRNA V3–V4 amplicon taxonomic profiling. This revealed elevated taxonomic diversity in field shoots over WMG, predominantly Proteobacteria succeeded by Actinobacteria, Firmicutes, Bacteroidetes, and 15 other phyla including several candidate phyla (135 families, 179 genera). Callus stocks also displayed broad bacterial diversity (16 phyla; 96 families; 141 genera) bearing resemblance to field tissues with Proteobacterial dominance but a reduction in its share, enrichment of Actinobacteria and Firmicutes, disappearance of some field-associated phyla and detection of a few additional taxonomic groups over field community. Similar results were documented during 16S V3–V4 amplicon taxonomic profiling on Thompson Seedless field shoot tip and callus tissues. Video microscopy on tissue homogenates corroborated enormous endophytic bacteria. This study elucidates a vast diversity of cultivation-recalcitrant endophytic bacteria prevailing in grapevine field shoots, their in vitro introduction, and unsuspecting sustenance with possible silent participation in tissue culture processes.




Similar content being viewed by others
Abbreviations
- FDW:
-
Filter-sterilized distilled water post autoclaving
- NA:
-
Nutrient agar
- SATS:
-
Spotting- and tilt-spreading
- SP-SDS:
-
Single plate-serial dilution spotting
- TSA:
-
Trypticase soy agar
- WMG:
-
Whole metagenome
References
Andreolli M, Lampis S, Zapparoli G, Angelini E, Vallini G (2016) Diversity of bacterial endophytes in 3 and 15 year-old grapevines of Vitis vinifera cv. Corvina and their potential for plant growth promotion and phytopathogen control. Microbiol Res 183:42–52
Ashelford KE, Weightman AJ, Fry JC (2002) PRIMROSE: a computer program for generating and estimating the phylogenetic range of 16S rRNA oligonucleotide probes and primers in conjunction with the RDP-II database. Nucleic Acids Res 30:3481–3489
Baldan E, Nigris S, Populin F, Zottini M, Squartini A, Baldan B (2014) Identification of culturable bacterial endophyte community isolated from tissues of Vitis vinifera “Glera”. Plant Biosyst 148:508–516
Bao E, Jiang T, Kaloshian I, Girke T (2011) SEED: efficient clustering of next-generation sequences. Bioinformatics 27:2502–2509
Berg G, Rybakova D, Grube M, Köberl M (2016) The plant microbiome explored: implications for experimental botany. J Exp Bot 77:995–1002
Browne HP, Forster SC, Anonye BO et al (2016) Culturing of ‘unculturable’ human microbiota reveals novel taxa and extensive sporulation. Nature 533:543–546
Bulgarelli D, Rott M, Schlaeppi K et al (2012) Revealing structure and assembly cues for the Arabidopsis root inhabiting bacterial microbiota. Nature 488:91–95
Bulgarelli D, Garrido-Oter R, Münch PC et al (2015) Structure and function of the bacterial root microbiota in wild and domesticated barley. Cell Host Microbe 17:392–403
Campisano A, Antonielli L, Pancher M, Yousaf S, Pindo M, Pertot I (2014) Bacterial endophytic communities in the grapevine depend on pest management. PLoS One 9:e112763
Compant S, Kaplan H, Sessitsch A, Nowak J, Ait Barka E, Clément C (2008) Endophytic colonization of Vitis vinifera L. by Burkholderia phytofirmans strain PsJN: from the rhizosphere to inflorescence tissues. FEMS Microbiol Ecol 63:84–93
Compant S, Mitter B, Colli-Mull JG, Gangl H, Sessitsch A (2011) Endophytes of grapevine flowers, berries and seeds: identification of cultivable bacteria, comparison with other plant parts, and visualization of niches of colonization. Microb Ecol 62:188–197
Compant S, Brader G, Muzammil S, Sessitsch A, Lebrihi A, Mathieu F (2013) Use of beneficial bacteria and their secondary metabolites to control grapevine pathogen diseases. Biocontrol 58:435–455
De Souza RS, Okura VK, Armanhi JS et al (2016) Unlocking the bacterial and fungal communities assemblages of sugarcane microbiome. Sci Rep 6:28774
Edwards J, Johnson C, Santos-Medellín C et al (2015) Structure, variation, and assembly of the root-associated microbiomes of rice. Proc Natl Acad Sci 112:E911–E920
Esposito-Polesi NP, de Abreu-Tarazi MF, de Almeida CV, Tsai SM, de Almeida M (2017) Investigation of endophytic bacterial community in supposedly axenic cultures of pineapple and orchids with evidence on abundant intracellular bacteria. Curr Microbiol 74:103–113
Goldammer T (2015) Grape grower’s handbook. Apex Publishers, Centreville
Goto K, Omura T, Hara Y, Sadaie Y (2000) Application of the partial 16S rDNA sequence as an index for rapid identification of species in the genus Bacillus. J Gen Appl Microbiol 46:1–8
Gouda S, Das G, Sen SK, Shin HS, Patra JK (2016) Endophytes: a treasure house of bioactive compounds of medicinal importance. Front Microbiol 7:1538
Hallmann J, Quadt-Hallmann A, Mahaffee WF, Kloepper JW (1997) Bacterial endophytes in agricultural crops. Can J Microbiol 43:895–914
Hardoim PR, van Overbeek LS, Berg G et al (2015) The hidden world within plants: ecological and evolutionary considerations for defining functioning of microbial endophytes. Microbiol Mol Biol Rev 79:293–320
Herman EB (2004) Recent advances in plant tissue culture VIII. Microbial contaminants in plant tissue cultures: solutions and opportunities 1996–2003. Agrictech Consultants Inc, Shrub Oak
Heuer H, Krsek M, Baker P, Smalla K, Wellington EMH (1997) Analysis of actinomycete communities by specific amplification of genes encoding 16S rRNA and gel-electrophoretic separation in denaturing gradients. Appl Environ Microbiol 63:3233–3241
Holland MA, Polacco JC (1994) PPFMs and other covert contaminants: is there more to plant physiology than just plant? Annu Rev Plant Physiol Plant Mol Biol 45:197–209
Jaillon O, Aury JM, Noel B et al (2007) The grapevine genome sequence suggests ancestral hexaploidization in major angiosperm phyla. Nature 449:463–467
Kane ME, Kauth P, Johnson T (2011) Culture indexing for bacterial and fungal contaminants. In: Trigiano RN, Gray DJ (eds) Plant tissue culture, development, and biotechnology. CRC Press, Boca Raton, pp 239–243
Kanehisa M, Goto S, Kawashima S, Okuno Y, Hattori M (2004) The KEGG resource for deciphering the genome. Nucleic Acids Res 32(suppl 1):D277–D280
Lundberg DS, Lebeis SL, Paredes SH et al (2012) Defining the core Arabidopsis thaliana root microbiome. Nature 488:86–90
Mühling M, Woolven-Allen J, Murrell JC, Joint I (2008) Improved group-specific PCR primers for denaturing gradient gel electrophoresis analysis of the genetic diversity of complex microbial communities. ISME J 2:379–392
Nehmé B, Gilbert Y, Létourneau V et al (2009) Culture-independent characterization of archaeal biodiversity in swine confinement building bioaerosols. Appl Environ Microbiol 75:5445–5450
O’Malley MA (2015) Endosymbiosis and its implications for evolutionary theory. Proc Natl Acad Sci USA 112:10270–10277
Orlikowska T, Nowak K, Reed B (2017) Bacteria in the plant tissue culture environment. Plant Cell Tissue Organ Cult 128:487–508
Pandey SS, Singh S, Babu CV, Shanker K, Srivastava NK, Kalra A (2016) Endophytes of opium poppy differentially modulate host plant productivity and genes for the biosynthetic pathway of benzylisoquinoline alkaloids. Planta 243:1097–1114
Péros JP, Torregrosa L, Berger G (1998) Variability among Vitis vinifera cultivars in micropropagation, organogenesis and antibiotic sensitivity. J Exp Bot 49:171–179
Pinto C, Pinho D, Sousa S, Pinheiro M, Egas C, Gomes AC (2014) Unravelling the diversity of grapevine microbiome. PLoS One 9:e85622
Podolich O, Ardanov P, Zaets I, Pirttilä AM, Kozyrovska N (2015) Reviving of the endophytic bacterial community as a putative mechanism of plant resistance. Plant Soil 388:367–377
Quambusch M, Pirttilä AM, Tejesvi MV, Winkelmann T, Bartsch M (2014) Endophytic bacteria in plant tissue culture: differences between easy- and difficult-to-propagate Prunus avium genotypes. Tree Physiol 34:524–533
Rappé MS, Giovannoni SJ (2003) The uncultured microbial majority. Annu Rev Microbiol 57:369–394
Roberts S, Kolewe M (2010) Plant natural products from cultured multipotent cells. Nat Biotechnol 28:1175–1176
Sessitsch A, Hardoim P, Döring J et al (2012) Functional characteristics of an endophyte community colonizing rice roots as revealed by metagenomic analysis. Mol Plant Microbe Interact 25:28–36
Shittu HO, Castroverde DC, Nazar RN, Robb J (2009) Plant-endophyte interplay protects tomato against a virulent Verticillium. Planta 229:415–426
Stach JEM, Maldonado LA, Ward AC, Goodfellow M, Bull AT (2003) New primers for the class Actinobacteria: application to marine and terrestrial environments. Environ Microbiol 5:828–841
Theodorakopoulos N, Bachar D, Christen R, Alain K, Chapon V (2013) Exploration of Deinococcus-Thermus molecular diversity by novel group-specific PCR primers. Microbiol Open 2:862–872
Thomas P (2004a) A three-step screening procedure for detection of covert and endophytic bacteria in plant tissue cultures. Curr Sci 87:67–72
Thomas P (2004b) In vitro decline in plant cultures: detection of a legion of covert bacteria as the cause for degeneration of long-term micropropagated triploid watermelon cultures. Plant Cell Tissue Organ Cult 77:173–179
Thomas P (2011) Intense association of non-culturable endophytic bacteria with antibiotic-cleansed in vitro watermelon and their activation in degenerating cultures. Plant Cell Rep 30:2313–2325
Thomas P, Reddy MK (2013) Microscopic elucidation of abundant endophytic bacteria colonizing the cell wall—plasma membrane peri-space in the shoot-tip tissue of banana. AoB Plants 5:plt011
Thomas P, Sekhar AC (2014) Live cell imaging reveals extensive intracellular cytoplasmic colonization of banana by normally non-cultivable endophytic bacteria. AoB Plants 6:plu002
Thomas P, Sekhar AC (2017) Cultivation versus molecular analysis of banana (Musa sp.) shoot-tip tissue reveals enormous diversity of normally uncultivable endophytic bacteria. Microb Ecol 73:885–899
Thomas P, Soly TA (2009) Endophytic bacteria associated with growing shoot tips of banana (Musa sp.) cv. Grand Naine and the affinity of endophytes to the host. Microb Ecol 58:952–964
Thomas P, Kumari S, Swarna GK, Prakash DP, Dinesh MR (2007) Ubiquitous presence of fastidious endophytic bacteria in field shoots and index-negative apparently clean shoot-tip cultures of papaya. Plant Cell Rep 26:1491–1499
Thomas P, Swarna GK, Patil P, Rawal RD (2008) Ubiquitous presence of normally non-culturable endophytic bacteria in field shoot-tips of banana and their gradual activation to quiescent cultivable form in tissue cultures. Plant Cell Tissue Organ Cult 93:39–54
Thomas P, Mujawar MM, Sekhar AC, Upreti R (2014) Physical impaction injury effects on bacterial cells during spread-plating influenced by cell characteristics of the organisms. J Appl Microbiol 116:911–922
Thomas P, Sekhar AC, Upreti R, Mujawar MM, Pasha SS (2015) Optimization of single plate-serial dilution spotting (SP-SDS) with sample anchoring as an assured method for bacterial and yeast cfu enumeration and single colony isolation from diverse samples. Biotechnol Rep 8:45–55
Upreti R, Thomas P (2015) Root-associated bacterial endophytes from Ralstonia solanacearum resistant and susceptible tomato cultivars and their pathogen antagonistic effects. Front Microbiol 6:255
Vandenkoornhuyse P, Quaiser A, Duhamel M, Van AL, Dufresne A (2015) The importance of the microbiome of the plant holobiont. New Phytol 206:1196–1206
Wang H, Liang X, Wan Q, Wang X, Bi Y (2009) Ethylene and nitric oxide are involved in maintaining ion homeostasis in Arabidopsis callus under salt stress. Planta 230:293–307
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Yousaf S, Bulgari D, Bergna A, Pancher M, Quaglino F, Casati P, Campisano A (2014) Pyrosequencing detects human and animal pathogenic taxa in the grapevine endosphere. Front Microbiol 5:327
Zarraonaindia I, Owens SM, Weisenhorn P et al (2015) The soil microbiome influences grapevine-associated microbiota. mBio 6:e02527–14
Zhang W, Curtin C, Kikuchi M, Franco C (2002) Integration of jasmonic acid and light irradiation for enhancement of anthocyanin biosynthesis in Vitis vinifera suspension cultures. Plant Sci 162:459–468
Acknowledgements
The study was funded partly under the ICAR-AMAAS Net-work project ‘Exploration of the endophytic microbial diversity in horticultural crops through metagenomics and cultivation’ funded through the ICAR-National Bureau of Agriculturally Important Microorganisms, Mau Nath Bhanjan, Uttar Pradesh, India and partly by ICAR-Indian Institute of Horticultural Research, Bengaluru, India under the sub-project: ‘Tissue culture systems in horticultural crops with reference to management and exploitation of endophytes’. The author thanks Dr. T. P. Rajendran (Former Assistant Director General-Plant Protection, ICAR and Acting Director, National Institute of Biotic Stress Management, Raipur, India) for the critical reading of the manuscript and the suggestions. This publication bears ICAR-IIHR Contribution No. 21/2017.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
425_2017_2733_MOESM2_ESM.mp4
Movie 01. Tissue homogenate from surface-sterilized field-shoot-tip tissues of grape ‘Flame Seedless’ showing abundant motile bacterial cocci and bacilli besides the tissue debris under bright field microscopy (1000×) (MP4 10590 kb)
425_2017_2733_MOESM3_ESM.mp4
Movie 02. Tissue homogenate from healthy 1 year plus old callus culture of grape ‘Flame Seedless’ showing abundant motile bacterial cocci and bacilli corresponding to bacteria under bright field microscopy (1000×) (MP4 4597 kb)
425_2017_2733_MOESM4_ESM.mp4
Movie 03. Pure culture of Pantoea ananatis (Proteobacterium) isolated from index-positive grape callus ‘Flame Seedless’ under bright field microscopy (1000×) showing condensed rods with motility (MP4 10209 kb)
425_2017_2733_MOESM5_ESM.mp4
Movie 04. Pure culture of Staphylococcus haemolyticus (Firmicute) isolated from index-positive grape callus ‘Flame Seedless’ under bright field microscopy (1000×) with small cocci displaying motility (MP4 8711 kb)
425_2017_2733_MOESM6_ESM.xls
Data set 1: Detailed path analysis WMG profiling on Flame Seedless field-shoot-tip functional analysis after filtering the contigs corresponding to Viridiplantae through MEGAN SEED classification (XLS 36 kb)
425_2017_2733_MOESM7_ESM.xls
Data set 2: Detailed path analysis WMG profiling on Flame Seedless field-shoot-tip functional analysis after filtering the contigs corresponding to Viridiplantae through KEGG pathway analysis (XLS 38 kb)
425_2017_2733_MOESM8_ESM.xls
Data set 3. Comparison of Flame Seedless and Thompson Seedless field and callus tissues for taxonomic diversity at family level (XLS 184 kb)
425_2017_2733_MOESM9_ESM.xls
Data set 4. Comparison of Flame Seedless and Thompson Seedless field and callus tissues for taxonomic diversity at genus level (XLS 183 kb)
Rights and permissions
About this article
Cite this article
Thomas, P., Sekhar, A.C. & Shaik, S.P. High taxonomic diversity of cultivation-recalcitrant endophytic bacteria in grapevine field shoots, their in vitro introduction, and unsuspected persistence. Planta 246, 879–898 (2017). https://doi.org/10.1007/s00425-017-2733-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00425-017-2733-5