Abstract
Objectives
To comprehensively summarize and meta-analyze the concurrence across voxel-based morphometric (VBM) neuroimaging studies of migraine.
Methods
Neuroimaging studies published from origin to August 1, 2021 were searched in six databases including PubMed, Web of Science, Excerpta Medica Database (EMBASE), China National Knowledge Infrastructure (CNKI), Wanfang Database, and Chongqing VIP. Study selection, quality assessment, and data extraction were conducted by two independent researchers. Anisotropic effect size-signed differential map** (AES-SDM) and activation likelihood estimation (ALE) were used to perform the meta-analysis of available studies reporting whole-brain gray matter (GM) structural data in migraine patients. Clinical variables correlation analysis and migraine subgroup analysis were also conducted.
Results
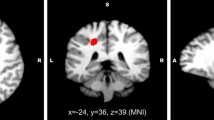
40 articles were included after the strict screening, containing 1616 migraine patients and 1681 matched healthy subjects (HS) in total. Using the method of AES-SDM, migraine patients showed GM increase in the bilateral amygdala, the bilateral parahippocampus, the bilateral temporal poles, the bilateral superior temporal gyri, the left hippocampus, the right superior frontal gyrus, and the left middle temporal gyrus, as well as GM decrease in the left insula, the bilateral cerebellum (hemispheric lobule IX), the right dorsal medulla, the bilateral rolandic operculum, the right middle frontal gyrus, and the right inferior parietal gyrus. Using the method of ALE, migraine patients showed GM increase in the left parahippocampus and GM decrease in the left insula. The results of correlation analysis showed that many of these brain regions were associated with migraine headache frequency and migraine disease duration. Migraine patients in different subtypes (such as migraine without aura (MwoA), migraine with aura (MwA), episodic migraine (EM), chronic migraine (CM), vestibular migraine (VM), etc.), and in different periods (in the ictal and interictal periods) presented not entirely consistent GM alterations.
Conclusion
Migraine patients have GM alterations in multiple brain regions associated with sensation, affection, cognition, and descending modulation aspects of pain. These changes might be a consequence of repeated migraine attacks. Further studies are required to determine how these GM changes can be used to diagnose, monitor disease progression, or exploit potential therapeutic interventions for migraine patients.



Similar content being viewed by others
Data availability statement
The datasets analyzed in the current study are available from the corresponding authors upon reasonable request.
References
Headache Classification Committee of the International Headache Society (IHS) (2018) The International Classification of Headache Disorders, 3rd edition. Cephalalgia 38(1):1–211. https://doi.org/10.1177/0333102417738202
Ashina M (2020) Migraine. N Engl J Med 383(19):1866–1876. https://doi.org/10.1056/NEJMra1915327
GBD (2016) Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100):1211–1259. https://doi.org/10.1016/S0140-6736(17)32154-2
GBD 2016 Headache Collaborators (2018) Global, regional, and national burden of migraine and tension-type headache, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol 17(11):954–976. https://doi.org/10.1016/S1474-4422(18)30322-3
Ashina M, Katsarava Z, Do TP et al (2021) Migraine: epidemiology and systems of care. Lancet 397(10283):1485–1495. https://doi.org/10.1016/S0140-6736(20)32160-7
Ashina M, Buse DC, Ashina H et al (2021) Migraine: integrated approaches to clinical management and emerging treatments. Lancet 397(10283):1505–1518. https://doi.org/10.1016/S0140-6736(20)32342-4
Shen FJ, Jia XU, Zhan YJ et al (2019) Acupuncture for migraine: a systematic review and meta-analysis. World J Acupunct-Mox 29(1):7–14. https://doi.org/10.1016/j.wjam.2019.03.004
Ashburner J, Friston KJ (2000) Voxel-based morphometry–the methods. Neuroimage 11(6 Pt 1):805–821. https://doi.org/10.1006/nimg.2000.0582
Matharu MS, Good CD, May A et al (2003) No change in the structure of the brain in migraine: a voxel-based morphometric study. Eur J Neurol 10(1):53–57. https://doi.org/10.1046/j.1468-1331.2003.00510.x
Rocca MA, Ceccarelli A, Falini A et al (2006) Brain gray matter changes in migraine patients with T2-visible lesions: a 3-T MRI study. Stroke 37(7):1765–1770. https://doi.org/10.1161/01.STR.0000226589.00599.4d
Coppola G, Petolicchio B, Di Renzo A et al (2017) Cerebral gray matter volume in patients with chronic migraine: correlations with clinical features. J Headache Pain 18(1):115. https://doi.org/10.1186/s10194-017-0825-z
Bonanno L, Lo Buono V, De Salvo S et al (2020) Brain morphologic abnormalities in migraine patients: an observational study. J Headache Pain 21(1):39. https://doi.org/10.1186/s10194-020-01109-2
Li Z, Zhou J, Lan L et al (2020) Concurrent brain structural and functional alterations in patients with migraine without aura: an fMRI study. J Headache Pain 21(1):141. https://doi.org/10.1186/s10194-020-01203-5
Yu Y, Zhao H, Dai L et al (2021) Headache frequency associates with brain microstructure changes in patients with migraine without aura. Brain Imaging Behav 15(1):60–67. https://doi.org/10.1007/s11682-019-00232-2
Müller VI, Cieslik EC, Laird AR et al (2018) Ten simple rules for neuroimaging meta-analysis. Neurosci Biobehav Rev 84:151–161. https://doi.org/10.1016/j.neubiorev.2017.11.012
Tahmasian M, Sepehry AA, Samea F et al (2019) Practical recommendations to conduct a neuroimaging meta-analysis for neuropsychiatric disorders. Hum Brain Mapp 40(17):5142–5154. https://doi.org/10.1002/hbm.24746
Radua J, Mataix-Cols D, Phillips ML et al (2012) A new meta-analytic method for neuroimaging studies that combines reported peak coordinates and statistical parametric maps. Eur Psychiatry 27(8):605–611. https://doi.org/10.1016/j.eurpsy.2011.04.001
Radua J, Rubia K, Canales-Rodríguez EJ et al (2014) Anisotropic kernels for coordinate-based meta-analyses of neuroimaging studies. Front Psychiatry 5:13. https://doi.org/10.3389/fpsyt.2014.00013
Eickhoff SB, Bzdok D, Laird AR et al (2012) Activation likelihood estimation meta-analysis revisited. Neuroimage 59(3):2349–2361. https://doi.org/10.1016/j.neuroimage.2011.09.017
Eickhoff SB, Laird AR, Grefkes C et al (2009) Coordinate-based activation likelihood estimation meta-analysis of neuroimaging data: a random-effects approach based on empirical estimates of spatial uncertainty. Hum Brain Mapp 30(9):2907–2926. https://doi.org/10.1002/hbm.20718
Turkeltaub PE, Eickhoff SB, Laird AR et al (2012) Minimizing within-experiment and within-group effects in Activation Likelihood Estimation meta-analyses. Hum Brain Mapp 33(1):1–13. https://doi.org/10.1002/hbm.21186
Albajes-Eizagirre A, Radua J (2018) What do results from coordinate-based meta-analyses tell us? Neuroimage 176:550–553. https://doi.org/10.1016/j.neuroimage.2018.04.065
Schmitz N, Admiraal-Behloul F, Arkink EB et al (2008) Attack frequency and disease duration as indicators for brain damage in migraine. Headache 48(7):1044–1055. https://doi.org/10.1111/j.1526-4610.2008.01133.x
Kim JH, Suh SI, Seol HY et al (2008) Regional grey matter changes in patients with migraine: a voxel-based morphometry study. Cephalalgia 28(6):598–604. https://doi.org/10.1111/j.1468-2982.2008.01550.x
Schmidt-Wilcke T, Gänssbauer S, Neuner T et al (2008) Subtle grey matter changes between migraine patients and healthy controls. Cephalalgia 28(1):1–4. https://doi.org/10.1111/j.1468-2982.2007.01428.x
Tessitore A, Russo A, Giordano A et al (2013) Disrupted default mode network connectivity in migraine without aura. J Headache Pain 14(1):89. https://doi.org/10.1186/1129-2377-14-89
Hubbard CS, Khan SA, Keaser ML et al (2014) Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 1(1):e20.14. https://doi.org/10.1523/ENEURO.0006-14.2014
Chanraud S, Di Scala G, Dilharreguy B et al (2014) Brain functional connectivity and morphology changes in medication-overuse headache: clue for dependence-related processes? Cephalalgia 34(8):605–615. https://doi.org/10.1177/0333102413519514
Obermann M, Wurthmann S, Steinberg BS et al (2014) Central vestibular system modulation in vestibular migraine. Cephalalgia 34(13):1053–1061. https://doi.org/10.1177/0333102414527650
Tessitore A, Russo A, Conte F et al (2015) Abnormal connectivity within executive resting-state network in migraine with aura. Headache 55(6):794–805. https://doi.org/10.1111/head.12587
Coppola G, Di Renzo A, Tinelli E et al (2015) Evidence for brain morphometric changes during the migraine cycle: a magnetic resonance-based morphometry study. Cephalalgia 35(9):783–791. https://doi.org/10.1177/0333102414559732
Liu J, Lan L, Mu J et al (2015) Genetic contribution of catechol-O-methyltransferase in hippocampal structural and functional changes of female migraine sufferers. Hum Brain Mapp 36(5):1782–1795. https://doi.org/10.1002/hbm.22737
Lai TH, Chou KH, Fuh JL et al (2016) Gray matter changes related to medication overuse in patients with chronic migraine. Cephalalgia 36(14):1324–1333. https://doi.org/10.1177/0333102416630593
Hougaard A, Amin FM, Arngrim N et al (2016) Sensory migraine aura is not associated with structural grey matter abnormalities. Neuroimage Clin 11:322–327. https://doi.org/10.1016/j.nicl.2016.02.007
Zhang J, Wu YL, Su J et al (2017) Assessment of gray and white matter structural alterations in migraineurs without aura. J Headache Pain 18(1):74. https://doi.org/10.1186/s10194-017-0783-5
Liu J, Mu J, Liu Q et al (2017) Brain structural properties predict psychologically mediated hypoalgesia in an 8-week sham acupuncture treatment for migraine. Hum Brain Mapp 38(9):4386–4397. https://doi.org/10.1002/hbm.23667
Messina R, Rocca MA, Colombo B et al (2017) Structural brain abnormalities in patients with vestibular migraine. J Neurol 264(2):295–303. https://doi.org/10.1007/s00415-016-8349-z
Neeb L, Bastian K, Villringer K et al (2017) Structural gray matter alterations in chronic migraine: implications for a progressive disease? Headache 57(3):400–416. https://doi.org/10.1111/head.13012
Arkink EB, Schmitz N, Schoonman GG et al (2017) The anterior hypothalamus in cluster headache. Cephalalgia 37(11):1039–1050. https://doi.org/10.1177/0333102416660550
Palm-Meinders IH, Arkink EB, Koppen H et al (2017) Volumetric brain changes in migraineurs from the general population. Neurology 89(20):2066–2074. https://doi.org/10.1212/WNL.0000000000004640
Chen WT, Chou KH, Lee PL et al (2018) Comparison of gray matter volume between migraine and “strict-criteria” tension-type headache. J Headache Pain 19(1):4. https://doi.org/10.1186/s10194-018-0834-6
Celle S, Créac’h C, Boutet C et al (2018) Elderly patients with ongoing migraine show reduced gray matter volume in second somatosensory cortex. J Oral Facial Pain Headache 32(1):67–74. https://doi.org/10.11607/ofph.1866
Messina R, Rocca MA, Colombo B et al (2018) Gray matter volume modifications in migraine: a cross-sectional and longitudinal study. Neurology 91(3):e280–e292. https://doi.org/10.1212/WNL.0000000000005819
Husøy AK, Håberg AK, Rimol LM et al (2019) Cerebral cortical dimensions in headache sufferers aged 50 to 66 years: a population-based imaging study in the Nord-Trøndelag Health Study (HUNT-MRI). Pain 160(7):1634–1643. https://doi.org/10.1097/j.pain.0000000000001550
Wei HL, Zhou X, Chen YC et al (2019) Impaired intrinsic functional connectivity between the thalamus and visual cortex in migraine without aura. J Headache Pain 20(1):116. https://doi.org/10.1186/s10194-019-1065-1
Yang FC, Chou KH, Lee PL et al (2019) Patterns of gray matter alterations in migraine and restless legs syndrome. Ann Clin Transl Neurol 6(1):57–67. https://doi.org/10.1002/acn3.680
Liu HY, Lee PL, Chou KH et al (2020) The cerebellum is associated with 2-year prognosis in patients with high-frequency migraine. J Headache Pain 21(1):29. https://doi.org/10.1186/s10194-020-01096-4
Zhe X, Zhang X, Chen L et al (2021) Altered gray matter volume and functional connectivity in patients with vestibular migraine. Front Neurosci 15:683802. https://doi.org/10.3389/fnins.2021.683802
Chou KH, Lee PL, Liang CS et al (2021) Identifying neuroanatomical signatures in insomnia and migraine comorbidity. Sleep. https://doi.org/10.1093/sleep/zsaa202
Masson R, Demarquay G, Meunier D et al (2021) Is migraine associated to brain anatomical alterations? New data and coordinate-based meta-analysis. Brain Topogr 34(3):384–401. https://doi.org/10.1007/s10548-021-00824-6
Zhao L (2011) Functional connectivity network involved in acupuncture along meridians based on fMRI Study. Chengdu University of Traditional Chinese Medicine.
Chen X (2014) Chronification of Migraine: a Clinical and Brain Gray Matter Structure Study. Chinese PLA Medical School
Yao Q (2017) Grey matter volume abnormality affected by mood disorder in migraine without aura - initial exploration. Shanghai Jiao Tong University.
Zhe X, Zhang X, Chen L et al (2018) Cerebral grey matter volume abnormalities in patients with vestibular migraine. Diagn Imaging Interv Radiol 27(6):428–432
Li M, Li X, Zhu W et al (2020) The study of correlations between structural changes of gray matter and cognitive decline in patients with migraine without aura. Radiologic practice 35(3):329–333
Wang J, Liu B, Yu D et al (2021) Voxel-based gray matter volume study in patients with vestibular migraine. Chinese journal of magnetic resonance imaging 12(3):67–70+88
Cheng S, Xu G, Zhou J et al (2020) A multimodal meta-analysis of structural and functional changes in the brain of tinnitus. Front Hum Neurosci 14:28. https://doi.org/10.3389/fnhum.2020.00028
Shepherd AM, Matheson SL, Laurens KR et al (2012) Systematic meta-analysis of insula volume in schizophrenia. Biol Psychiatry 72(9):775–784. https://doi.org/10.1016/j.biopsych.2012.04.020
Du M, Liu J, Chen Z et al (2014) Brain grey matter volume alterations in late-life depression. J Psychiatry Neurosci 39(6):397–406. https://doi.org/10.1503/jpn.130275
Eickhoff SB, Nichols TE, Laird AR et al (2016) Behavior, sensitivity, and power of activation likelihood estimation characterized by massive empirical simulation. Neuroimage 137:70–85. https://doi.org/10.1016/j.neuroimage.2016.04.072
Dai Z, Zhong J, **ao P et al (2015) Gray matter correlates of migraine and gender effect: a meta-analysis of voxel-based morphometry studies. Neuroscience 299:88–96. https://doi.org/10.1016/j.neuroscience.2015.04.066
Jia Z, Yu S (2017) Grey matter alterations in migraine: a systematic review and meta-analysis. Neuroimage Clin 14:130–140. https://doi.org/10.1016/j.nicl.2017.01.019
Hu W, Guo J, Chen N et al (2015) A meta-analysis of voxel-based morphometric studies on migraine. Int J Clin Exp Med 8(3):4311–4319
Wang HZ, Wang WH, Shi HC et al (2020) Is there a reliable brain morphological signature for migraine? J Headache Pain 21(1):89. https://doi.org/10.1186/s10194-020-01158-7
Tracey I, Mantyh PW (2007) The cerebral signature for pain perception and its modulation. Neuron 55(3):377–391. https://doi.org/10.1016/j.neuron.2007.07.012
Veinante P, Yalcin I, Barrot M (2013) The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 1(1):9. https://doi.org/10.1186/2049-9256-1-9
Namkung H, Kim SH, Sawa A (2017) The insula: an underestimated brain area in clinical neuroscience, psychiatry, and neurology. Trends Neurosci 40(4):200–207. https://doi.org/10.1016/j.tins.2017.02.002
Moulton EA, Schmahmann JD, Becerra L et al (2010) The cerebellum and pain: passive integrator or active participator? Brain Res Rev 65(1):14–27. https://doi.org/10.1016/j.brainresrev.2010.05.005
Fassbender C, Murphy K, Foxe JJ et al (2004) A topography of executive functions and their interactions revealed by functional magnetic resonance imaging. Brain Res Cogn Brain Res 20(2):132–143. https://doi.org/10.1016/j.cogbrainres.2004.02.007
May A (2008) Chronic pain may change the structure of the brain. Pain 137(1):7–15. https://doi.org/10.1016/j.pain.2008.02.034
Liu MG, Chen J (2009) Roles of the hippocampal formation in pain information processing. Neurosci Bull 25(5):237–266. https://doi.org/10.1007/s12264-009-0905-4
Grant JA, Courtemanche J, Duerden EG et al (2010) Cortical thickness and pain sensitivity in zen meditators. Emotion 10(1):43–53. https://doi.org/10.1037/a0018334
Moulton EA, Becerra L, Maleki N et al (2011) Painful heat reveals hyperexcitability of the temporal pole in interictal and ictal migraine States. Cereb Cortex 21(2):435–448. https://doi.org/10.1093/cercor/bhq109
Mălîia MD, Donos C, Barborica A et al (2018) Functional map** and effective connectivity of the human operculum. Cortex 109:303–321. https://doi.org/10.1016/j.cortex.2018.08.024
Zhuo M (2017) Descending facilitation. Mol Pain 13:1744806917699212. https://doi.org/10.1177/1744806917699212
Khera T, Rangasamy V (2021) Cognition and pain: a review. Front Psychol 12:673962. https://doi.org/10.3389/fpsyg.2021.673962
Insausti R, Juottonen K, Soininen H et al (1998) MR volumetric analysis of the human entorhinal, perirhinal, and temporopolar cortices. AJNR Am J Neuroradiol 19(4):659–671
Smith AP, Henson RN, Dolan RJ et al (2004) fMRI correlates of the episodic retrieval of emotional contexts. Neuroimage 22(2):868–878. https://doi.org/10.1016/j.neuroimage.2004.01.049
Levy I, Hasson U, Avidan G et al (2001) Center-periphery organization of human object areas. Nat Neurosci 4(5):533–539. https://doi.org/10.1038/87490
Epstein R, Kanwisher N (1998) A cortical representation of the local visual environment. Nature 392(6676):598–601. https://doi.org/10.1038/33402
Arnott SR, Cant JS, Dutton GN et al (2008) Crinkling and crumpling: an auditory fMRI study of material properties. Neuroimage 43(2):368–378. https://doi.org/10.1016/j.neuroimage.2008.07.033
Engelien A, Tüscher O, Hermans W et al (2006) Functional neuroanatomy of non-verbal semantic sound processing in humans. J Neural Transm (Vienna) 113(5):599–608. https://doi.org/10.1007/s00702-005-0342-0
Buse DC, Silberstein SD, Manack AN et al (2013) Psychiatric comorbidities of episodic and chronic migraine. J Neurol 260(8):1960–1969. https://doi.org/10.1007/s00415-012-6725-x
Minen MT, Begasse De Dhaem O, Kroon Van Diest A et al (2016) Migraine and its psychiatric comorbidities. J Neurol Neurosurg Psychiatry 87(7):741–749. https://doi.org/10.1136/jnnp-2015-312233
Brooks JC, Zambreanu L, Godinez A et al (2005) Somatotopic organisation of the human insula to painful heat studied with high resolution functional imaging. Neuroimage 27(1):201–209. https://doi.org/10.1016/j.neuroimage.2005.03.041
Henderson LA, Gandevia SC, Macefield VG (2007) Somatotopic organization of the processing of muscle and cutaneous pain in the left and right insula cortex: a single-trial fMRI study. Pain 128(1–2):20–30. https://doi.org/10.1016/j.pain.2006.08.013
Garcia-Larrea L, Peyron R (2013) Pain matrices and neuropathic pain matrices: a review. Pain 154(Suppl 1):S29-s43. https://doi.org/10.1016/j.pain.2013.09.001
Iannetti GD, Mouraux A (2010) From the neuromatrix to the pain matrix (and back). Exp Brain Res 205(1):1–12. https://doi.org/10.1007/s00221-010-2340-1
Segerdahl AR, Mezue M, Okell TW et al (2015) The dorsal posterior insula subserves a fundamental role in human pain. Nat Neurosci 18(4):499–500. https://doi.org/10.1038/nn.3969
Frot M, Faillenot I, Mauguière F (2014) Processing of nociceptive input from posterior to anterior insula in humans. Hum Brain Mapp 35(11):5486–5499. https://doi.org/10.1002/hbm.22565
Gaist D, Hougaard A, Garde E et al (2018) Migraine with visual aura associated with thicker visual cortex. Brain 141(3):776–785. https://doi.org/10.1093/brain/awx382
Özkan E, Gürsoy-Özdemir Y (2021) Occipital bending in migraine with visual aura. Headache 61(10):1562–1567. https://doi.org/10.1111/head.14240
Borsook D, Upadhyay J, Chudler EH et al (2010) A key role of the basal ganglia in pain and analgesia–insights gained through human functional imaging. Mol Pain 6:27. https://doi.org/10.1186/1744-8069-6-27
Yuan K, Zhao L, Cheng P et al (2013) Altered structure and resting-state functional connectivity of the basal ganglia in migraine patients without aura. J Pain 14(8):836–844. https://doi.org/10.1016/j.jpain.2013.02.010
Grahn JA, Parkinson JA, Owen AM (2008) The cognitive functions of the caudate nucleus. Prog Neurobiol 86(3):141–155. https://doi.org/10.1016/j.pneurobio.2008.09.004
Bednarczyk EM, Remler B, Weikart C et al (1998) Global cerebral blood flow, blood volume, and oxygen metabolism in patients with migraine headache. Neurology 50(6):1736–1740. https://doi.org/10.1212/wnl.50.6.1736
Longoni M, Ferrarese C (2006) Inflammation and excitotoxicity: role in migraine pathogenesis. Neurol Sci 27(Suppl 2):S107-110. https://doi.org/10.1007/s10072-006-0582-2
Pietrobon D, Striessnig J (2003) Neurobiology of migraine. Nat Rev Neurosci 4(5):386–398. https://doi.org/10.1038/nrn1102
Shin JH, Kim YK, Kim HJ et al (2014) Altered brain metabolism in vestibular migraine: comparison of interictal and ictal findings. Cephalalgia 34(1):58–67. https://doi.org/10.1177/0333102413498940
Deutschländer A, Hüfner K, Kalla R et al (2008) Unilateral vestibular failure suppresses cortical visual motion processing. Brain 131:1025–1034. https://doi.org/10.1093/brain/awn035
Bense S, Deutschländer A, Stephan T et al (2004) Preserved visual-vestibular interaction in patients with bilateral vestibular failure. Neurology 63(1):122–128. https://doi.org/10.1212/01.wnl.0000129545.79566.6a
Brandt T, Bartenstein P, Janek A et al (1998) Reciprocal inhibitory visual-vestibular interaction. Visual motion stimulation deactivates the parieto-insular vestibular cortex. Brain 121(9):1749–1758. https://doi.org/10.1093/brain/121.9.1749
Bostan AC, Strick PL (2018) The basal ganglia and the cerebellum: nodes in an integrated network. Nat Rev Neurosci 19(6):338–350. https://doi.org/10.1038/s41583-018-0002-7
Bingel U, Gläscher J, Weiller C et al (2004) Somatotopic representation of nociceptive information in the putamen: an event-related fMRI study. Cereb Cortex 14(12):1340–1345. https://doi.org/10.1093/cercor/bhh094
Valentine JC, Pigott TD, Rothstein HR (2010) How many studies do you need?: A primer on statistical power for meta-analysis. J Educ Behav Stat 35(2):215–247. https://doi.org/10.3102/1076998609346961
Jackson D, Turner R (2017) Power analysis for random-effects meta-analysis. Res Synth Methods 8(3):290–302. https://doi.org/10.1002/jrsm.1240
Acknowledgements
The authors thank Yuke Teng and Lin Yang for their assistance and suggestions.
Funding
The study is supported by funds from National Science Fund for Distinguished Young Scholars (No. 82225050), National Natural Science Foundation of China (No. 81973958), Sichuan Province Scientific and Technological Innovation Team for Youths (No. 2019JDTD0011) and Innovation Team and Talents Cultivation Program of National Administration of Traditional Chinese Medicine (No: ZYYCXTD-D-202003). The sponsors play no part in study design, data collection, management, and analysis.
Author information
Authors and Affiliations
Contributions
ZL and FZ contributed to the study conception and design and conceived the data analysis strategy. XZ, MG, YC and XL acquired the data. XZ, JZ, MG and NJ collated and analyzed the data. XZ, JZ and MG drafted the manuscript. SC, SH, ZT, ZL and FZ discussed, read, and revised the manuscript. All authors approved the publication of this manuscript.
Corresponding authors
Ethics declarations
Conflicts of interest
The authors declare that they have no conflicts of interest.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Zhang, X., Zhou, J., Guo, M. et al. A systematic review and meta-analysis of voxel-based morphometric studies of migraine. J Neurol 270, 152–170 (2023). https://doi.org/10.1007/s00415-022-11363-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-022-11363-w