Abstract
Objectives
To determine the clinical, anatomical, genetic and pathological features of dual frontotemporal lobar degeneration (FTLD) pathology: FTLD-tau and FTLD-TDP-43 in a large clinicopathological cohort.
Methods
We selected subjects with mixed FTLD-TDP and FTLD-tau from 247 FTLD cases from the University of California, San Francisco, Neurodegenerative Disease Brain Bank collected between 2000 and 2016 and compared their clinical, anatomical, genetic, imaging and pathological signatures with those of subjects with pure FTLD.
Results
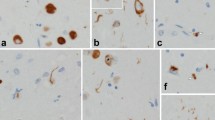
We found nine cases (3.6%) with prominent FTLD-TDP and FTLD-tau. Six cases were sporadic, whereas one case had a C9ORF72 expansion, another had a TARDBP A90V variant, and the other had an MAPT p.A152T variant. The subtypes of FTLD-TDP and FTLD-tau varied. Mixed FTLD cases were older and tended to show a higher burden of Alzheimer disease pathology (3/9, 33%). The neuroimaging signature of mixed cases, in general, included more widespread atrophy than that of pure groups. Specifically, cases of mixed corticobasal degeneration (CBD) with FTLD-TDP showed more prominent asymmetric left-sided atrophy than did those of pure CBD. However, the clinical phenotype of mixed cases was similar to that seen in pure FTLD.
Conclusions
Although patients with mixed FTLD-TDP and FTLD-tau are rare, in-depth clinical, pathological and genetic investigations may shed light on the genetic and biochemical pathways that cause the accumulation of multiple proteinaceous inclusions and inform therapeutic targets that may be beneficial to each one of these abnormal protein misfoldings.



Similar content being viewed by others
References
McKhann GM, Albert MS, Grossman M et al (2001) Clinical and pathological diagnosis of frontotemporal dementia: report of the Work Group on Frontotemporal Dementia and Pick’s Disease. Arch Neurol 58(11):1803–1809
Mackenzie I, Neumann M, Bigio E et al (2010) Nomenclature and nosology for neuropathologic subtypes of frontotemporal lobar degeneration: an update. Acta Neuropathol 119(1):1–4
Baborie A, Griffiths TD, Jaros E et al (2011) Pathological correlates of frontotemporal lobar degeneration in the elderly. Acta Neuropathol 121(3):365–371
Kovacs GG (2015) Invited review: Neuropathology of tauopathies: principles and practice. Neuropathol Appl Neurobiol 41(1):3–23
Mackenzie IR, Neumann M, Baborie A et al (2011) A harmonized classification system for FTLD-TDP pathology. Acta Neuropathol 122(1):111–113
Nascimento C, Di Lorenzo Alho AT, Bazan Conceição Amaral C et al (2017) Prevalence of transactive response DNA-binding protein 43 (TDP-43) proteinopathy in cognitively normal older adults: systematic review and meta-analysis. Neuropathol Appl Neurobiol 44(3):286–297
Cairns NJ, Bigio EH, Mackenzie IR et al (2007) Neuropathologic diagnostic and nosologic criteria for frontotemporal lobar degeneration: consensus of the Consortium for Frontotemporal Lobar Degeneration. Acta Neuropathol 114(1):5–22
Flanagan EP, Duffy JR, Whitwell JL et al (2016) Mixed tau and TDP-43 pathology in a patient with unclassifiable primary progressive aphasia. Neurocase 22(1):55–59
Freeman SH, Spires-Jones T, Hyman BT, Growdon JH, Frosch MP (2008) TAR-DNA binding protein 43 in Pick disease. J Neuropathol Exp Neurol 67(1):62–67
Koga S, Sanchez-Contreras M, Josephs KA et al (2016) Distribution and characteristics of transactive response DNA binding protein 43 kDa pathology in progressive supranuclear palsy. Mov Disord 32(2):246–255
Uryu K, Nakashima-Yasuda H, Forman MS et al (2008) Concomitant TAR-DNA-binding protein 43 pathology is present in Alzheimer disease and corticobasal degeneration but not in other tauopathies. J Neuropathol Exp Neurol 67(6):555–564
Winton MJ, Van Deerlin VM, Kwong LK et al (2008) A90V TDP-43 variant results in the aberrant localization of TDP-43 in vitro. FEBS Lett 582(15):2252–2256
Lee SE, Tartaglia MC, Yener G et al (2013) Neurodegenerative disease phenotypes in carriers of MAPT p.A152T, a risk factor for frontotemporal dementia spectrum disorders and Alzheimer disease. Alzheimer Dis Assoc Disord 27(4):302–309
Montine TJ, Phelps CH, Beach TG et al (2012) National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol 123(1):1–11
Rodriguez RD, Suemoto CK, Molina M et al (2016) Argyrophilic Grain Disease: Demographics, Clinical, and Neuropathological Features From a Large Autopsy Study. J Neuropathol Exp Neurol 75(7):628–635
Crary JF, Trojanowski JQ, Schneider JA et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128(6):755–766
Kovacs GG, Ferrer I, Grinberg LT et al (2016) Aging-related tau astrogliopathy (ARTAG): harmonized evaluation strategy. Acta Neuropathol 131(1):87–102
Coppola G, Chinnathambi S, Lee JJ et al (2012) Evidence for a role of the rare p.A152T variant in MAPT in increasing the risk for FTD-spectrum and Alzheimer’s diseases. Hum Mol Genet 21(15):3500–3512
Josephs KA, Hodges JR, Snowden JS et al (2011) Neuropathological background of phenotypical variability in frontotemporal dementia. Acta Neuropathol 122(2):137–153
Kovacs GG, Molnár K, László L et al (2011) A peculiar constellation of tau pathology defines a subset of dementia in the elderly. Acta Neuropathol 122(2):205–222
Robinson AC, Thompson JC, Weedon L et al (2014) No interaction between tau and TDP-43 pathologies in either frontotemporal lobar degeneration or motor neurone disease. Neuropathol Appl Neurobiol 40(7):844–854
Storey K, Johanidesová S, Matěj R et al (2016) FTLD-TDP and progressive supranuclear palsy in comorbidity-a report of two cases with different clinical presentations. Neurocase 1–7
Yokota O, Davidson Y, Bigio EH et al (2010) Phosphorylated TDP-43 pathology and hippocampal sclerosis in progressive supranuclear palsy. Acta Neuropathol 120(1):55–66
Bieniek KF, Murray ME, Rutherford NJ et al (2013) Tau pathology in frontotemporal lobar degeneration with C9ORF72 hexanucleotide repeat expansion. Acta Neuropathol 125(2):289–302
Hsiung GY, DeJesus-Hernandez M, Feldman HH et al (2012) Clinical and pathological features of familial frontotemporal dementia caused by C9ORF72 mutation on chromosome 9p. Brain 135(Pt 3):709–722
King A, Al-Sarraj S, Troakes C et al (2013) Mixed tau, TDP-43 and p62 pathology in FTLD associated with a C9ORF72 repeat expansion and p.Ala239Thr MAPT (tau) variant. Acta Neuropathol 125(2):303–310
Amador-Ortiz C, Lin WL, Ahmed Z et al (2007) TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol 61(5):435–445
Josephs KA, Murray ME, Whitwell JL et al (2016) Updated TDP-43 in Alzheimer’s disease staging scheme. Acta Neuropathol 131(4):571–585
Josephs KA, Whitwell JL, Knopman DS et al (2008) Abnormal TDP-43 immunoreactivity in AD modifies clinicopathologic and radiologic phenotype. Neurology 70(19 Pt 2):1850–1857
Bigio EH, Mishra M, Hatanpaa KJ et al (2010) TDP-43 pathology in primary progressive aphasia and frontotemporal dementia with pathologic Alzheimer disease. Acta Neuropathol 120(1):43–54
Davidson YS, Raby S, Foulds PG et al (2011) TDP-43 pathological changes in early onset familial and sporadic Alzheimer’s disease, late onset Alzheimer’s disease and Down’s syndrome: association with age, hippocampal sclerosis and clinical phenotype. Acta Neuropathol 122(6):703–713
Josephs KA, Whitwell JL, Weigand SD et al (2014) TDP-43 is a key player in the clinical features associated with Alzheimer’s disease. Acta Neuropathol 127(6):811–824
Dickson DW, Davies P, Bevona C et al (1994) Hippocampal sclerosis: a common pathological feature of dementia in very old (> or = 80 years of age) humans. Acta Neuropathol 88(3):212–221
Kouri N, Oshima K, Takahashi M et al (2013) Corticobasal degeneration with olivopontocerebellar atrophy and TDP-43 pathology: an unusual clinicopathologic variant of CBD. Acta Neuropathol 125(5):741–752
Kovacs GG, Wöhrer A, Ströbel T et al (2011) Unclassifiable tauopathy associated with an A152T variation in MAPT exon 7. Clin Neuropathol 30(1):3–10
Graff-Radford J, Whitwell JL, Dickson DW, Josephs KA (2013) Pallidonigroluysian atrophy associated with p.A152T variant in MAPT. Parkinsonism Relat Disord 19(9):838–841
Kara E, Ling H, Pittman AM et al (2012) The MAPT p.A152T variant is a risk factor associated with tauopathies with atypical clinical and neuropathological features. Neurobiol Aging 33(9):2231.e7-.e14
Labbé C, Ogaki K, Lorenzo-Betancor O et al (2015) Role for the microtubule-associated protein tau variant p.A152T in risk of α-synucleinopathies. Neurology 85(19):1680–1686
Nag S, Yu L, Capuano AW et al (2015) Hippocampal sclerosis and TDP-43 pathology in aging and Alzheimer disease. Ann Neurol 77(6):942–952
Acknowledgments
This study was supported by the Financial Supporting Project of Long-term Overseas Dispatch of PNU's Tenure-track Faculty, 2015 to Eun-Joo Kim.
Funding
Funding was provided by the NIH Grant #K24AG053435, P50AG023501 and P01AG019724, Rainwater Foundation to Lea T. Grinberg.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors have nothing to disclose.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kim, EJ., Brown, J.A., Deng, J. et al. Mixed TDP-43 proteinopathy and tauopathy in frontotemporal lobar degeneration: nine case series. J Neurol 265, 2960–2971 (2018). https://doi.org/10.1007/s00415-018-9086-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-018-9086-2