Abstract
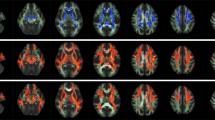
Using advanced MRI techniques, we investigated the presence and topographical distribution of brain grey matter (GM) and white matter (WM) alterations in dominant optic atrophy (DOA) patients with genetically proven OPA1 mutation as well as their correlation with clinical and neuro-ophthalmologic findings. Nineteen DOA patients underwent neurological, neuro-ophthalmologic and brainstem auditory evoked potentials (BAEP) evaluations. Voxel-wise methods were applied to assess regional GM and WM abnormalities in patients compared to 20 healthy controls. Visual acuity was reduced in 16 patients. Six DOA patients (4 with missense mutations) had an abnormal I peripheral component (auditory nerve) at BAEP. Compared to controls, DOA patients had significant atrophy of the optic nerves (p < 0.0001). Voxel-based morphometry (VBM) analysis showed that, compared to controls, DOA patients had significant WM atrophy of the chiasm and optic tracts; whereas no areas of GM atrophy were found. Tract-based spatial statistics (TBSS) analysis showed that compared to controls, DOA patients had significantly lower mean diffusivity, axial and radial diffusivity in the WM of the cerebellum, brainstem, thalamus, fronto-occipital-temporal lobes, including the cingulum, corpus callosum, corticospinal tract and optic radiation bilaterally. No abnormalities of fractional anisotropy were detected. No correlations were found between volumetric and diffusivity abnormalities quantified with MRI and clinical and neuro-ophthalmologic measures of disease severity. Consistently with pathological studies, tissue loss in DOA patients is limited to anterior optic pathways reflecting retinal ganglion cell degeneration. Distributed abnormalities of diffusivity indexes might reflect abnormal intracellular mitochondrial morphology as well as alteration of protein levels due to OPA1 mutations.



Similar content being viewed by others
References
Yu-Wai-Man P, Griffiths PG, Burke A, Sellar PW, Clarke MP, Gnanaraj L, Ah-Kine D, Hudson G, Czermin B, Taylor RW, Horvath R, Chinnery PF (2010) The prevalence and natural history of dominant optic atrophy due to OPA1 mutations. Ophthalmology 117(8):1538–1546. doi:10.1016/j.ophtha.2009.12.038
Johnston PB, Gaster RN, Smith VC, Tripathi RC (1979) A clinicopathologic study of autosomal dominant optic atrophy. Am J Ophthalmol 88(5):868–875
Kjer P, Jensen OA, Klinken L (1983) Histopathology of eye, optic nerve and brain in a case of dominant optic atrophy. Acta Ophthalmol (Copenh) 61(2):300–312
Alexander C, Votruba M, Pesch UE, Thiselton DL, Mayer S, Moore A, Rodriguez M, Kellner U, Leo-Kottler B, Auburger G, Bhattacharya SS, Wissinger B (2000) OPA1, encoding a dynamin-related GTPase, is mutated in autosomal dominant optic atrophy linked to chromosome 3q28. Nat Genet 26(2):211–215. doi:10.1038/79944
Delettre C, Lenaers G, Griffoin JM, Gigarel N, Lorenzo C, Belenguer P, Pelloquin L, Grosgeorge J, Turc-Carel C, Perret E, Astarie-Dequeker C, Lasquellec L, Arnaud B, Ducommun B, Kaplan J, Hamel CP (2000) Nuclear gene OPA1, encoding a mitochondrial dynamin-related protein, is mutated in dominant optic atrophy. Nat Genet 26(2):207–210. doi:10.1038/79936
Amati-Bonneau P, Valentino ML, Reynier P, Gallardo ME, Bornstein B, Boissiere A, Campos Y, Rivera H, de la Aleja JG, Carroccia R, Iommarini L, Labauge P, Figarella-Branger D, Marcorelles P, Furby A, Beauvais K, Letournel F, Liguori R, La Morgia C, Montagna P, Liguori M, Zanna C, Rugolo M, Cossarizza A, Wissinger B, Verny C, Schwarzenbacher R, Martin MA, Arenas J, Ayuso C, Garesse R, Lenaers G, Bonneau D, Carelli V (2008) OPA1 mutations induce mitochondrial DNA instability and optic atrophy ‘plus’ phenotypes. Brain 131(Pt 2):338–351. doi:10.1093/brain/awm298
Hudson G, Amati-Bonneau P, Blakely EL, Stewart JD, He L, Schaefer AM, Griffiths PG, Ahlqvist K, Suomalainen A, Reynier P, McFarland R, Turnbull DM, Chinnery PF, Taylor RW (2008) Mutation of OPA1 causes dominant optic atrophy with external ophthalmoplegia, ataxia, deafness and multiple mitochondrial DNA deletions: a novel disorder of mtDNA maintenance. Brain 131(Pt 2):329–337. doi:10.1093/brain/awm272
Zanna C, Ghelli A, Porcelli AM, Karbowski M, Youle RJ, Schimpf S, Wissinger B, Pinti M, Cossarizza A, Vidoni S, Valentino ML, Rugolo M, Carelli V (2008) OPA1 mutations associated with dominant optic atrophy impair oxidative phosphorylation and mitochondrial fusion. Brain 131(Pt 2):352–367. doi:10.1093/brain/awm335
Yu-Wai-Man P, Griffiths PG, Gorman GS, Lourenco CM, Wright AF, Auer-Grumbach M, Toscano A, Musumeci O, Valentino ML, Caporali L, Lamperti C, Tallaksen CM, Duffey P, Miller J, Whittaker RG, Baker MR, Jackson MJ, Clarke MP, Dhillon B, Czermin B, Stewart JD, Hudson G, Reynier P, Bonneau D, Marques W Jr, Lenaers G, McFarland R, Taylor RW, Turnbull DM, Votruba M, Zeviani M, Carelli V, Bindoff LA, Horvath R, Amati-Bonneau P, Chinnery PF (2010) Multi-system neurological disease is common in patients with OPA1 mutations. Brain 133(Pt 3):771–786. doi:10.1093/brain/awq007
Audoin B, Fernando KT, Swanton JK, Thompson AJ, Plant GT, Miller DH (2006) Selective magnetization transfer ratio decrease in the visual cortex following optic neuritis. Brain 129(Pt 4):1031–1039. doi:10.1093/brain/awl039
Ciccarelli O, Toosy AT, Hickman SJ, Parker GJ, Wheeler-Kingshott CA, Miller DH, Thompson AJ (2005) Optic radiation changes after optic neuritis detected by tractography-based group map**. Hum Brain Mapp 25(3):308–316. doi:10.1002/hbm.20101
Yucel YH, Zhang Q, Weinreb RN, Kaufman PL, Gupta N (2003) Effects of retinal ganglion cell loss on magno-, parvo-, koniocellular pathways in the lateral geniculate nucleus and visual cortex in glaucoma. Prog Retin Eye Res 22(4):465–481 (S1350946203000260 [pii])
Kitajima M, Korogi Y, Hirai T, Hamatake S, Ikushima I, Sugahara T, Shigematsu Y, Takahashi M, Mukuno K (1997) MR changes in the calcarine area resulting from retinal degeneration. AJNR Am J Neuroradiol 18(7):1291–1295
Inglese M, Rovaris M, Bianchi S, La Mantia L, Mancardi GL, Ghezzi A, Montagna P, Salvi F, Filippi M (2001) Magnetic resonance imaging, magnetisation transfer imaging, and diffusion weighted imaging correlates of optic nerve, brain, and cervical cord damage in Leber’s hereditary optic neuropathy. J Neurol Neurosurg Psychiatry 70(4):444–449
Rocca MA, Valsasina P, Pagani E, Bianchi-Marzoli S, Milesi J, Falini A, Comi G, Filippi M (2011) Extra-visual functional and structural connection abnormalities in leber’s hereditary optic neuropathy. PLoS One 6(2):e17081. doi:10.1371/journal.pone.0017081
Rocca MA, Mesaros S, Preziosa P, Pagani E, Stosic-Opincal T, Dujmovic-Basuroski I, Drulovic J, Filippi M (2013) Wallerian and trans-synaptic degeneration contribute to optic radiation damage in multiple sclerosis: a diffusion tensor MRI study. Mult Scler. doi:10.1177/1352458513485146
Smith SM, Zhang Y, Jenkinson M, Chen J, Matthews PM, Federico A, De Stefano N (2002) Accurate, robust, and automated longitudinal and cross-sectional brain change analysis. NeuroImage 17(1):479–489 (S1053811902910402 [pii])
Barcella V, Rocca MA, Bianchi-Marzoli S, Milesi J, Melzi L, Falini A, Pierro L, Filippi M (2010) Evidence for retrochiasmatic tissue loss in Leber’s hereditary optic neuropathy. Hum Brain Mapp 31(12):1900–1906. doi:10.1002/hbm.20985
Riccitelli G, Rocca MA, Pagani E, Rodegher ME, Rossini PM, Falini A, Comi GC, Filippi M (2011) Cognitive impairment in multiple sclerosis is associated to different patterns of gray matter atrophy according to clinical phenotype. Hum Brain Mapp 32(10):1535–1543
Ashburner J (2007) A fast diffeomorphic image registration algorithm. NeuroImage 38(1):95–113. doi:10.1016/j.neuroimage.2007.07.007
Basser PJ, Mattiello J, LeBihan D (1994) Estimation of the effective self-diffusion tensor from the NMR spin echo. J Magn Reson B 103(3):247–254
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage 31(4):1487–1505. doi:10.1016/j.neuroimage.2006.02.024
Nichols TE, Holmes AP (2002) Nonparametric permutation tests for functional neuroimaging: a primer with examples. Hum Brain Mapp 15(1):1–25. doi:10.1002/hbm.1058
Smith SM, Nichols TE (2009) Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localisation in cluster inference. NeuroImage 44(1):83–98. doi:10.1016/j.neuroimage.2008.03.061
Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PC, Mori S (2008) Tract probability maps in stereotaxic spaces: analyses of white matter anatomy and tract-specific quantification. NeuroImage 39(1):336–347. doi:10.1016/j.neuroimage.2007.07.053
Wakana S, Caprihan A, Panzenboeck MM, Fallon JH, Perry M, Gollub RL, Hua K, Zhang J, Jiang H, Dubey P, Blitz A, van Zijl P, Mori S (2007) Reproducibility of quantitative tractography methods applied to cerebral white matter. NeuroImage 36(3):630–644. doi:10.1016/j.neuroimage.2007.02.049
Eickhoff SB, Heim S, Zilles K, Amunts K (2006) Testing anatomically specified hypotheses in functional imaging using cytoarchitectonic maps. NeuroImage 32(2):570–582. doi:10.1016/j.neuroimage.2006.04.204
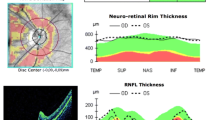
Barboni P, Savini G, Parisi V, Carbonelli M, La Morgia C, Maresca A, Sadun F, De Negri AM, Carta A, Sadun AA, Carelli V (2011) Retinal nerve fiber layer thickness in dominant optic atrophy measurements by optical coherence tomography and correlation with age. Ophthalmology 118(10):2076–2080. doi:10.1016/j.ophtha.2011.02.027
Barboni P, Savini G, Cascavilla ML, Caporali L, Milesi J, Borrelli E, La Morgia C, Valentino ML, Triolo G, Lembo A, Carta A, De Negri A, Sadun F, Rizzo G, Parisi V, Pierro L, Marzoli SB, Zeviani M, Sadun AA, Bandello F, Carelli V (2014) Early macular retinal ganglion cell loss in dominant optic atrophy: genotype-phenotype correlation. Am J Ophthalmol. doi:10.1016/j.ajo.2014.05.034
Votruba M, Leary S, Losseff N, Bhattacharya SS, Moore AT, Miller DH, Moseley IF (2000) MRI of the intraorbital optic nerve in patients with autosomal dominant optic atrophy. Neuroradiology 42(3):180–183
Barboni P, Carbonelli M, Savini G, Foscarini B, Parisi V, Valentino ML, Carta A, De Negri A, Sadun F, Zeviani M, Sadun AA, Schimpf S, Wissinger B, Carelli V (2010) OPA1 mutations associated with dominant optic atrophy influence optic nerve head size. Ophthalmology 117(8):1547–1553. doi:10.1016/j.ophtha.2009.12.042
Leruez S, Milea D, Defoort-Dhellemmes S, Colin E, Crochet M, Procaccio V, Ferre M, Lamblin J, Drouin V, Vincent-Delorme C, Lenaers G, Hamel C, Blanchet C, Juul G, Larsen M, Verny C, Reynier P, Amati-Bonneau P, Bonneau D (2013) Sensorineural hearing loss in OPA1-linked disorders. Brain 136(Pt 7):e236. doi:10.1093/brain/aws340
Bette S, Zimmermann U, Wissinger B, Knipper M (2007) OPA1, the disease gene for optic atrophy type Kjer, is expressed in the inner ear. Histochem Cell Biol 128(5):421–430. doi:10.1007/s00418-007-0321-7
Rance G, Kearns LS, Tan J, Gravina A, Rosenfeld L, Henley L, Carew P, Graydon K, O’Hare F, Mackey DA (2012) Auditory function in individuals within Leber’s hereditary optic neuropathy pedigrees. J Neurol 259(3):542–550. doi:10.1007/s00415-011-6230-7
Rance G, Fava R, Baldock H, Chong A, Barker E, Corben L, Delatycki MB (2008) Speech perception ability in individuals with Friedreich ataxia. Brain 131(Pt 8):2002–2012. doi:10.1093/brain/awn104
Maresca A, la Morgia C, Caporali L, Valentino ML, Carelli V (2013) The optic nerve: a “mito-window” on mitochondrial neurodegeneration. Molecular and cellular neurosciences 55:62–76. doi:10.1016/j.mcn.2012.08.004
Bette S, Schlaszus H, Wissinger B, Meyermann R, Mittelbronn M (2005) OPA1, associated with autosomal dominant optic atrophy, is widely expressed in the human brain. Acta Neuropathol 109(4):393–399. doi:10.1007/s00401-004-0970-8
Cogliati S, Frezza C, Soriano ME, Varanita T, Quintana-Cabrera R, Corrado M, Cipolat S, Costa V, Casarin A, Gomes LC, Perales-Clemente E, Salviati L, Fernandez-Silva P, Enriquez JA, Scorrano L (2013) Mitochondrial cristae shape determines respiratory chain supercomplexes assembly and respiratory efficiency. Cell 155(1):160–171. doi:10.1016/j.cell.2013.08.032
Lenaers G, Reynier P, Elachouri G, Soukkarieh C, Olichon A, Belenguer P, Baricault L, Ducommun B, Hamel C, Delettre C (2009) OPA1 functions in mitochondria and dysfunctions in optic nerve. Int J Biochem Cell Biol 41(10):1866–1874. doi:10.1016/j.biocel.2009.04.013
Milesi J, Rocca MA, Bianchi-Marzoli S, Petrolini M, Pagani E, Falini A, Comi G, Filippi M (2012) Patterns of white matter diffusivity abnormalities in Leber’s hereditary optic neuropathy: a tract-based spatial statistics study. J Neurol 259(9):1801–1807. doi:10.1007/s00415-011-6406-1
Rizzo G, Tozer KR, Tonon C, Manners D, Testa C, Malucelli E, Valentino ML, La Morgia C, Barboni P, Randhawa RS, Ross-Cisneros FN, Sadun AA, Carelli V, Lodi R (2012) Secondary post-geniculate involvement in Leber’s hereditary optic neuropathy. PLoS One 7(11):e50230. doi:10.1371/journal.pone.0050230
Milea D, Sander B, Wegener M, Jensen H, Kjer B, Jorgensen TM, Lund-Andersen H, Larsen M (2010) Axonal loss occurs early in dominant optic atrophy. Acta Ophthalmol 88(3):342–346. doi:10.1111/j.1755-3768.2008.01469.x
Acknowledgements
E.L. is supported by Pierfranco e Luisa Mariani Foundation, Italy. We thank Franco Carrara, Melissa Petrolini and Elisabetta Pagani for their contribution to the analysis of the data.
Conflicts of interest
The authors declare that they have no conflict of interest.
Ethical standard
This study was performed in accordance with the ethical standards laid down in the 1965 Declaration of Helsinski and its later amendments.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Rocca, M.A., Bianchi-Marzoli, S., Messina, R. et al. Distributed abnormalities of brain white matter architecture in patients with dominant optic atrophy and OPA1 mutations. J Neurol 262, 1216–1227 (2015). https://doi.org/10.1007/s00415-015-7696-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-015-7696-5