Abstract
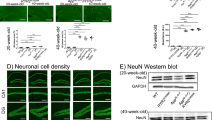
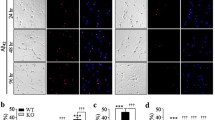
The mechanisms underlying ryanodine receptor (RyR) dysfunction associated with Alzheimer disease (AD) are still not well understood. Here, we show that neuronal RyR2 channels undergo post-translational remodeling (PKA phosphorylation, oxidation, and nitrosylation) in brains of AD patients, and in two murine models of AD (3 × Tg-AD, APP +/− /PS1 +/−). RyR2 is depleted of calstabin2 (KFBP12.6) in the channel complex, resulting in endoplasmic reticular (ER) calcium (Ca2+) leak. RyR-mediated ER Ca2+ leak activates Ca2+-dependent signaling pathways, contributing to AD pathogenesis. Pharmacological (using a novel RyR stabilizing drug Rycal) or genetic rescue of the RyR2-mediated intracellular Ca2+ leak improved synaptic plasticity, normalized behavioral and cognitive functions and reduced Aβ load. Genetically altered mice with congenitally leaky RyR2 exhibited premature and severe defects in synaptic plasticity, behavior and cognitive function. These data provide a mechanism underlying leaky RyR2 channels, which could be considered as potential AD therapeutic targets.







Similar content being viewed by others
References
Adasme T, Haeger P, Paula-Lima AC, Espinoza I, Casas-Alarcon MM, Carrasco MA, Hidalgo C (2011) Involvement of ryanodine receptors in neurotrophin-induced hippocampal synaptic plasticity and spatial memory formation. Proc Natl Acad Sci USA 108:3029–3034. doi:10.1073/pnas.1013580108
Allen PB, Ouimet CC, Greengard P (1997) Spinophilin, a novel protein phosphatase 1 binding protein localized to dendritic spines. Proc Natl Acad Sci USA 94:9956–9961
Andersson DC, Betzenhauser MJ, Reiken S, Meli AC, Umanskaya A, **e W, Shiomi T, Zalk R, Lacampagne A, Marks AR (2011) Ryanodine receptor oxidation causes intracellular calcium leak and muscle weakness in aging. Cell Metab 14:196–207. doi:10.1016/j.cmet.2011.05.014
Barelli H, Lebeau A, Vizzavona J, Delaere P, Chevallier N, Drouot C, Marambaud P, Ancolio K, Buxbaum JD, Khorkova O et al (1997) Characterization of new polyclonal antibodies specific for 40 and 42 amino acid-long amyloid beta peptides: their use to examine the cell biology of presenilins and the immunohistochemistry of sporadic Alzheimer’s disease and cerebral amyloid angiopathy cases. Mol Med 3:695–707
Bennett DA, Yu L, Yang J, Srivastava GP, Aubin C, De Jager PL (2015) Epigenomics of Alzheimer’s disease. Transl Res 165:200–220. doi:10.1016/j.trsl.2014.05.006
Berridge MJ (2011) Calcium signalling and Alzheimer’s disease. Neurochem Res 36:1149–1156. doi:10.1007/s11064-010-0371-4
Branca C, Wisely EV, Hartman LK, Caccamo A, Oddo S (2014) Administration of a selective beta2 adrenergic receptor antagonist exacerbates neuropathology and cognitive deficits in a mouse model of Alzheimer’s disease. Neurobiol Aging 35:2726–2735. doi:10.1016/j.neurobiolaging.2014.06.011
Briggs CA, Schneider C, Richardson JC, Stutzmann GE (2013) beta amyloid peptide plaques fail to alter evoked neuronal calcium signals in APP/PS1 Alzheimer’s disease mice. Neurobiol Aging 34:1632–1643. doi:10.1016/j.neurobiolaging.2012.12.013
Brillantes AB, Ondrias K, Scott A, Kobrinsky E, Ondriasova E, Moschella MC, Jayaraman T, Landers M, Ehrlich BE, Marks AR (1994) Stabilization of calcium release channel (ryanodine receptor) function by FK506-binding protein. Cell 77:513–523
Bruno AM, Huang JY, Bennett DA, Marr RA, Hastings ML, Stutzmann GE (2012) Altered ryanodine receptor expression in mild cognitive impairment and Alzheimer’s disease. Neurobiol Aging 33(1001):e1001–e1006. doi:10.1016/j.neurobiolaging.2011.03.011
Chakroborty S, Briggs C, Miller MB, Goussakov I, Schneider C, Kim J, Wicks J, Richardson JC, Conklin V, Cameransi BG et al (2012) Stabilizing ER Ca(2+) Channel Function as an Early Preventative Strategy for Alzheimer’s Disease. PLoS One 7:e52056. doi:10.1371/journal.pone.0052056
Chakroborty S, Kim J, Schneider C, Jacobson C, Molgo J, Stutzmann GE (2012) Early presynaptic and postsynaptic calcium signaling abnormalities mask underlying synaptic depression in presymptomatic Alzheimer’s disease mice. J Neurosci 32:8341–8353. doi:10.1523/JNEUROSCI.0936-12.2012
Chakroborty S, Stutzmann GE (2014) Calcium channelopathies and Alzheimer’s disease: insight into therapeutic success and failures. Eur J Pharmacol 739:83–95. doi:10.1016/j.ejphar.2013.11.012
Chan SL, Mayne M, Holden CP, Geiger JD, Mattson MP (2000) Presenilin-1 mutations increase levels of ryanodine receptors and calcium release in PC12 cells and cortical neurons. J Biol Chem 275:18195–18200. doi:10.1074/jbc.M000040200
Cheung KH, Shineman D, Muller M, Cardenas C, Mei L, Yang J, Tomita T, Iwatsubo T, Lee VM, Foskett JK (2008) Mechanism of Ca2+ disruption in Alzheimer’s disease by presenilin regulation of InsP3 receptor channel gating. Neuron 58:871–883. doi:10.1016/j.neuron.2008.04.015
Colquhoun D, Hawkes AG (1982) On the stochastic properties of bursts of single ion channel openings and of clusters of bursts. Philos Trans R Soc Lond B Biol Sci 300:1–59
Copello JA, Barg S, Onoue H, Fleischer S (1997) Heterogeneity of Ca2+ gating of skeletal muscle and cardiac ryanodine receptors. Biophys J 73:141–156. doi:10.1016/S0006-3495(97)78055-X
Crary JF, Trojanowski JQ, Schneider JA, Abisambra JF, Abner EL, Alafuzoff I, Arnold SE, Attems J, Beach TG, Bigio EH et al (2014) Primary age-related tauopathy (PART): a common pathology associated with human aging. Acta Neuropathol 128:755–766. doi:10.1007/s00401-014-1349-0
Dang V, Medina B, Das D, Moghadam S, Martin KJ, Lin B, Naik P, Patel D, Nosheny R, Wesson Ashford J et al (2014) Formoterol, a long-acting beta2 adrenergic agonist, improves cognitive function and promotes dendritic complexity in a mouse model of Down syndrome. Biol Psychiatry 75:179–188. doi:10.1016/j.biopsych.2013.05.024
Del Prete D, Checler F, Chami M (2014) Ryanodine receptors: physiological function and deregulation in Alzheimer disease. Mol Neurodegener 9:21. doi:10.1186/1750-1326-9-21
Dobarro M, Gerenu G, Ramirez MJ (2013) Propranolol reduces cognitive deficits, amyloid and tau pathology in Alzheimer’s transgenic mice. Int J Neuropsychopharmacol 16:2245–2257. doi:10.1017/S1461145713000631
Duff K, Eckman C, Zehr C, Yu X, Prada CM, Perez-tur J, Hutton M, Buee L, Harigaya Y, Yager D et al (1996) Increased amyloid-beta42(43) in brains of mice expressing mutant presenilin 1. Nature 383:710–713. doi:10.1038/383710a0
Echeverria V, Ducatenzeiler A, Chen CH, Cuello AC (2005) Endogenous beta-amyloid peptide synthesis modulates cAMP response element-regulated gene expression in PC12 cells. Neuroscience 135:1193–1202. doi:10.1016/j.neuroscience.2005.06.057
Etcheberrigaray R, Hirashima N, Nee L, Prince J, Govoni S, Racchi M, Tanzi RE, Alkon DL (1998) Calcium responses in fibroblasts from asymptomatic members of Alzheimer’s disease families. Neurobiol Dis 5:37–45. doi:10.1006/nbdi.1998.0176
Etkin A, Alarcon JM, Weisberg SP, Touzani K, Huang YY, Nordheim A, Kandel ER (2006) A role in learning for SRF: deletion in the adult forebrain disrupts LTD and the formation of an immediate memory of a novel context. Neuron 50:127–143. doi:10.1016/j.neuron.2006.03.013
Feng J, Yan Z, Ferreira A, Tomizawa K, Liauw JA, Zhuo M, Allen PB, Ouimet CC, Greengard P (2000) Spinophilin regulates the formation and function of dendritic spines. Proc Natl Acad Sci USA 97:9287–9292
Flammang B, Pardossi-Piquard R, Sevalle J, Debayle D, Dabert-Gay AS, Thevenet A, Lauritzen I, Checler F (2012) Evidence that the amyloid-beta protein precursor intracellular domain, AICD, derives from beta-secretase-generated C-terminal fragment. J Alzheimers Dis 30:145–153. doi:10.3233/JAD-2012-112186
Flucher BE, Andrews SB, Fleischer S, Marks AR, Caswell A, Powell JA (1993) Triad formation: organization and function of the sarcoplasmic reticulum calcium release channel and triadin in normal and dysgenic muscle in vitro. J Cell Biol 123:1161–1174
Francis YI, Fa M, Ashraf H, Zhang H, Staniszewski A, Latchman DS, Arancio O (2009) Dysregulation of histone acetylation in the APP/PS1 mouse model of Alzheimer’s disease. J Alzheimers Dis 18:131–139. doi:10.3233/JAD-2009-1134
Gapp K, Woldemichael BT, Bohacek J, Mansuy IM (2014) Epigenetic regulation in neurodevelopment and neurodegenerative diseases. Neuroscience 264:99–111. doi:10.1016/j.neuroscience.2012.11.040
Ghosh D, LeVault KR, Barnett AJ, Brewer GJ (2012) A reversible early oxidized redox state that precedes macromolecular ROS damage in aging nontransgenic and 3 × Tg-AD mouse neurons. J Neurosci 32:5821–5832. doi:10.1523/JNEUROSCI.6192-11.2012
Giannini G, Conti A, Mammarella S, Scrobogna M, Sorrentino V (1995) The ryanodine receptor/calcium channel genes are widely and differentially expressed in murine brain and peripheral tissues. J Cell Biol 128:893–904
Gong B, Cao Z, Zheng P, Vitolo OV, Liu S, Staniszewski A, Moolman D, Zhang H, Shelanski M, Arancio O (2006) Ubiquitin hydrolase Uch-L1 rescues beta-amyloid-induced decreases in synaptic function and contextual memory. Cell 126:775–788. doi:10.1016/j.cell.2006.06.046
Goslin K (1991) Rat hippocampal neurons in low-density culture. MIT Press, Cambridge, pp 251–281
Goussakov I, Miller MB, Stutzmann GE (2010) NMDA-mediated Ca(2 +) influx drives aberrant ryanodine receptor activation in dendrites of young Alzheimer’s disease mice. J Neurosci 30:12128–12137. doi:10.1523/JNEUROSCI.2474-10.2010
Heck A, Fastenrath M, Coynel D, Auschra B, Bickel H, Freytag V, Gschwind L, Hartmann F, Jessen F, Kaduszkiewicz Het al (2015) Genetic analysis of association between calcium signaling and hippocampal activation, memory performance in the young and old, and risk for sporadic Alzheimer disease. JAMA Psychiatry. doi:10.1001/jamapsychiatry.2015.1309
Hidalgo C, Carrasco MA (2011) Redox control of brain calcium in health and disease. Antioxid Redox Signal 14:1203–1207. doi:10.1089/ars.2010.3711
Hsiao K, Chapman P, Nilsen S, Eckman C, Harigaya Y, Younkin S, Yang F, Cole G (1996) Correlative memory deficits, Abeta elevation, and amyloid plaques in transgenic mice. Science 274:99–102
Igbavboa U, Johnson-Anuna LN, Rossello X, Butterick TA, Sun GY, Wood WG (2006) Amyloid beta-protein1-42 increases cAMP and apolipoprotein E levels which are inhibited by beta1 and beta2-adrenergic receptor antagonists in mouse primary astrocytes. Neuroscience 142:655–660. doi:10.1016/j.neuroscience.2006.06.056
Lalonde R, Kim HD, Maxwell JA, Fukuchi K (2005) Exploratory activity and spatial learning in 12-month-old APP(695)SWE/co+ PS1/DeltaE9 mice with amyloid plaques. Neurosci Lett 390:87–92. doi:10.1016/j.neulet.2005.08.028
Lauritzen I, Pardossi-Piquard R, Bauer C, Brigham E, Abraham JD, Ranaldi S, Fraser P, St-George-Hyslop P, Le Thuc O, Espin V et al (2012) The beta-secretase-derived C-terminal fragment of betaAPP, C99, but not Abeta, is a key contributor to early intraneuronal lesions in triple-transgenic mouse hippocampus. J Neurosci 32:16243–16255a. doi:10.1523/JNEUROSCI.2775-12.2012
LeDoux JE (2000) Emotion circuits in the brain. Annu Rev Neurosci 23:155–184. doi:10.1146/annurev.neuro.23.1.155
Leissring MA, Murphy MP, Mead TR, Akbari Y, Sugarman MC, Jannatipour M, Anliker B, Muller U, Saftig P, De Strooper B et al (2002) A physiologic signaling role for the gamma -secretase-derived intracellular fragment of APP. Proc Natl Acad Sci USA 99:4697–4702. doi:10.1073/pnas.072033799
Leissring MA, Parker I, LaFerla FM (1999) Presenilin-2 mutations modulate amplitude and kinetics of inositol 1, 4,5-trisphosphate-mediated calcium signals. J Biol Chem 274:32535–32538
Li R, Lindholm K, Yang LB, Yue X, Citron M, Yan R, Beach T, Sue L, Sabbagh M, Cai H et al (2004) Amyloid beta peptide load is correlated with increased beta-secretase activity in sporadic Alzheimer’s disease patients. Proc Natl Acad Sci USA 101:3632–3637. doi:10.1073/pnas.0205689101
Li S, ** M, Zhang D, Yang T, Koeglsperger T, Fu H, Selkoe DJ (2013) Environmental novelty activates beta2-adrenergic signaling to prevent the impairment of hippocampal LTP by Abeta oligomers. Neuron 77:929–941. doi:10.1016/j.neuron.2012.12.040
Liang L, Wei H (2015) Dantrolene, a treatment for Alzheimer disease? Alzheimer Dis Assoc Disord 29:1–5. doi:10.1097/WAD.0000000000000076
Liu X, Betzenhauser MJ, Reiken S, Meli AC, **e W, Chen BX, Arancio O, Marks AR (2012) Role of leaky neuronal ryanodine receptors in stress- induced cognitive dysfunction. Cell 150:1055–1067. doi:10.1016/j.cell.2012.06.052
Marambaud P, Ancolio K, Alves da Costa C, Checler F (1999) Effect of protein kinase A inhibitors on the production of Abeta40 and Abeta42 by human cells expressing normal and Alzheimer’s disease-linked mutated betaAPP and presenilin 1. Br J Pharmacol 126:1186–1190. doi:10.1038/sj.bjp.0702406
Marks AR (1997) Intracellular calcium-release channels: regulators of cell life and death. Am J Physiol 272:H597–H605
Marx SO, Reiken S, Hisamatsu Y, Gaburjakova M, Gaburjakova J, Yang YM, Rosemblit N, Marks AR (2001) Phosphorylation-dependent regulation of ryanodine receptors: a novel role for leucine/isoleucine zippers. J Cell Biol 153:699–708
Marx SO, Reiken S, Hisamatsu Y, Jayaraman T, Burkhoff D, Rosemblit N, Marks AR (2000) PKA phosphorylation dissociates FKBP12.6 from the calcium release channel (ryanodine receptor): defective regulation in failing hearts. Cell 101:365–376
Moolman DL, Vitolo OV, Vonsattel JP, Shelanski ML (2004) Dendrite and dendritic spine alterations in Alzheimer models. J Neurocytol 33:377–387. doi:10.1023/B:NEUR.0000044197.83514.64
Ni Y, Zhao X, Bao G, Zou L, Teng L, Wang Z, Song M, **ong J, Bai Y, Pei G (2006) Activation of beta2-adrenergic receptor stimulates gamma-secretase activity and accelerates amyloid plaque formation. Nat Med 12:1390–1396. doi:10.1038/nm1485
Nicholls RE, Alarcon JM, Malleret G, Carroll RC, Grody M, Vronskaya S, Kandel ER (2008) Transgenic mice lacking NMDAR-dependent LTD exhibit deficits in behavioral flexibility. Neuron 58:104–117. doi:10.1016/j.neuron.2008.01.039
Niu Y, Su Z, Zhao C, Song B, Zhang X, Zhao N, Shen X, Gong Y (2009) Effect of amyloid beta on capacitive calcium entry in neural 2a cells. Brain Res Bull 78:152–157. doi:10.1016/j.brainresbull.2008.10.003
Oddo S, Caccamo A, Shepherd JD, Murphy MP, Golde TE, Kayed R, Metherate R, Mattson MP, Akbari Y, LaFerla FM (2003) Triple-transgenic model of Alzheimer’s disease with plaques and tangles: intracellular Abeta and synaptic dysfunction. Neuron 39:409–421
Oules B, Del Prete D, Greco B, Zhang X, Lauritzen I, Sevalle J, Moreno S, Paterlini-Brechot P, Trebak M, Checler F et al (2012) Ryanodine receptor blockade reduces amyloid-beta load and memory impairments in Tg2576 mouse model of Alzheimer disease. J Neurosci 32:11820–11834. doi:10.1523/JNEUROSCI.0875-12.2012
Palavicini JP, Wang H, Bianchi E, Xu S, Rao JS, Kang DE, Lakshmana MK (2013) RanBP9 aggravates synaptic damage in the mouse brain and is inversely correlated to spinophilin levels in Alzheimer’s brain synaptosomes. Cell Death Dis 4:e667. doi:10.1038/cddis.2013.183
Paula-Lima AC, Adasme T, SanMartin C, Sebollela A, Hetz C, Carrasco MA, Ferreira ST, Hidalgo C (2011) Amyloid beta-peptide oligomers stimulate RyR-mediated Ca2+ release inducing mitochondrial fragmentation in hippocampal neurons and prevent RyR-mediated dendritic spine remodeling produced by BDNF. Antioxid Redox Signal 14:1209–1223. doi:10.1089/ars.2010.3287
Paula-Lima AC, Hidalgo C (2013) Amyloid beta-peptide oligomers, ryanodine receptor-mediated Ca(2+) release, and Wnt-5a/Ca(2+) signaling: opposing roles in neuronal mitochondrial dynamics? Front Cell Neurosci 7:120. doi:10.3389/fncel.2013.00120
Paylor R, Tracy R, Wehner J, Rudy JW (1994) DBA/2 and C57BL/6 mice differ in contextual fear but not auditory fear conditioning. Behav Neurosci 108:810–817
Peng J, Liang G, Inan S, Wu Z, Joseph DJ, Meng Q, Peng Y, Eckenhoff MF, Wei H (2012) Dantrolene ameliorates cognitive decline and neuropathology in Alzheimer triple transgenic mice. Neurosci Lett 516:274–279. doi:10.1016/j.neulet.2012.04.008
Pilpel Y, Segal M (2004) Activation of PKC induces rapid morphological plasticity in dendrites of hippocampal neurons via Rac and Rho-dependent mechanisms. Eur J Neurosci 19:3151–3164. doi:10.1111/j.0953-816X.2004.03380.x
Popugaeva E, Bezprozvanny I (2013) Role of endoplasmic reticulum Ca2+ signaling in the pathogenesis of Alzheimer disease. Front Mol Neurosci 6:29. doi:10.3389/fnmol.2013.00029
Prapong T, Uemura E, Hsu WH (2001) G protein and cAMP-dependent protein kinase mediate amyloid beta-peptide inhibition of neuronal glucose uptake. Experimental neurology 167:59–64. doi:10.1006/exnr.2000.7519
Reyes M, Stanton PK (1996) Induction of hippocampal long-term depression requires release of Ca2+ from separate presynaptic and postsynaptic intracellular stores. J Neurosci 16:5951–5960
Rosenberg PB, Mielke MM, Tschanz J, Cook L, Corcoran C, Hayden KM, Norton M, Rabins PV, Green RC, Welsh-Bohmer KA et al (2008) Effects of cardiovascular medications on rate of functional decline in Alzheimer disease. Am J Geriatr Psychiatry 16:883–892. doi:10.1097/JGP.0b013e318181276a
Saito K, Elce JS, Hamos JE, Nixon RA (1993) Widespread activation of calcium-activated neutral proteinase (calpain) in the brain in Alzheimer disease: a potential molecular basis for neuronal degeneration. Proc Natl Acad Sci USA 90:2628–2632
Santulli G, Marks AR (2015) Essential Roles of intracellular calcium release channels in muscle, brain, metabolism, and aging. Curr Mol Pharmacol 8:206–222
Schubert V, Da Silva JS, Dotti CG (2006) Localized recruitment and activation of RhoA underlies dendritic spine morphology in a glutamate receptor-dependent manner. J Cell Biol 172:453–467. doi:10.1083/jcb.200506136
Shan J, Betzenhauser MJ, Kushnir A, Reiken S, Meli AC, Wronska A, Dura M, Chen BX, Marks AR (2010) Role of chronic ryanodine receptor phosphorylation in heart failure and beta-adrenergic receptor blockade in mice. J Clin Invest 120:4375–4387. doi:10.1172/JCI37649
Shan J, Kushnir A, Betzenhauser MJ, Reiken S, Li J, Lehnart SE, Lindegger N, Mongillo M, Mohler PJ, Marks AR (2010) Phosphorylation of the ryanodine receptor mediates the cardiac fight or flight response in mice. J Clin Invest 120:4388–4398. doi:10.1172/JCI32726
Shilling D, Muller M, Takano H, Mak DO, Abel T, Coulter DA, Foskett JK (2014) Suppression of InsP3 receptor-mediated Ca2+ signaling alleviates mutant presenilin-linked familial Alzheimer’s disease pathogenesis. J Neurosci 34:6910–6923. doi:10.1523/JNEUROSCI.5441-13.2014
Shrestha BR, Vitolo OV, Joshi P, Lordkipanidze T, Shelanski M, Dunaevsky A (2006) Amyloid beta peptide adversely affects spine number and motility in hippocampal neurons. Mol Cell Neurosci 33:274–282. doi:10.1016/j.mcn.2006.07.011
Smith DL, Pozueta J, Gong B, Arancio O, Shelanski M (2009) Reversal of long-term dendritic spine alterations in Alzheimer disease models. Proc Natl Acad Sci USA 106:16877–16882. doi:10.1073/pnas.0908706106
Stutzmann GE, Caccamo A, LaFerla FM, Parker I (2004) Dysregulated IP3 signaling in cortical neurons of knock-in mice expressing an Alzheimer’s-linked mutation in presenilin1 results in exaggerated Ca2+ signals and altered membrane excitability. J Neurosci 24:508–513. doi:10.1523/JNEUROSCI.4386-03.2004
Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I (2006) Enhanced ryanodine receptor recruitment contributes to Ca2+ disruptions in young, adult, and aged Alzheimer’s disease mice. J Neurosci 26:5180–5189. doi:10.1523/JNEUROSCI.0739-06.2006
Supnet C, Grant J, Kong H, Westaway D, Mayne M (2006) Amyloid-beta-(1-42) increases ryanodine receptor-3 expression and function in neurons of TgCRND8 mice. J Biol Chem 281:38440–38447. doi:10.1074/jbc.M606736200
Tashiro A, Minden A, Yuste R (2000) Regulation of dendritic spine morphology by the rho family of small GTPases: antagonistic roles of Rac and Rho. Cereb Cortex 10:927–938
Texido L, Martin-Satue M, Alberdi E, Solsona C, Matute C (2011) Amyloid beta peptide oligomers directly activate NMDA receptors. Cell Calcium 49:184–190. doi:10.1016/j.ceca.2011.02.001
Unni VK, Zakharenko SS, Zablow L, DeCostanzo AJ, Siegelbaum SA (2004) Calcium release from presynaptic ryanodine-sensitive stores is required for long-term depression at hippocampal CA3-CA3 pyramidal neuron synapses. J Neurosci 24:9612–9622. doi:10.1523/JNEUROSCI.5583-03.2004
von Bernhardi R, Eugenin J (2012) Alzheimer’s disease: redox dysregulation as a common denominator for diverse pathogenic mechanisms. Antioxid Redox Signal 16:974–1031. doi:10.1089/ars.2011.4082
Wang D, Govindaiah G, Liu R, De Arcangelis V, Cox CL, **ang YK (2010) Binding of amyloid beta peptide to beta2 adrenergic receptor induces PKA-dependent AMPA receptor hyperactivity. FASEB J 24:3511–3521. doi:10.1096/fj.10-156661
Wehrens XH, Lehnart SE, Reiken SR, Deng SX, Vest JA, Cervantes D, Coromilas J, Landry DW, Marks AR (2004) Protection from cardiac arrhythmia through ryanodine receptor-stabilizing protein calstabin2. Science 304:292–296. doi:10.1126/science.1094301
Wisely EV, **ang YK, Oddo S (2014) Genetic suppression of beta2-adrenergic receptors ameliorates tau pathology in a mouse model of tauopathies. Hum Mol Genet 23:4024–4034. doi:10.1093/hmg/ddu116
Wu B, Yamaguchi H, Lai FA, Shen J (2013) Presenilins regulate calcium homeostasis and presynaptic function via ryanodine receptors in hippocampal neurons. Proc Natl Acad Sci USA 110:15091–15096. doi:10.1073/pnas.1304171110
Yang LB, Lindholm K, Yan R, Citron M, **a W, Yang XL, Beach T, Sue L, Wong P, Price D et al (2003) Elevated beta-secretase expression and enzymatic activity detected in sporadic Alzheimer disease. Nat Med 9:3–4. doi:10.1038/nm0103-3
Yu JT, Tan L, Ou JR, Zhu JX, Liu K, Song JH, Sun YP (2008) Polymorphisms at the beta2-adrenergic receptor gene influence Alzheimer’s disease susceptibility. Brain Res 1210:216–222. doi:10.1016/j.brainres.2008.03.019
Yu NN, Wang XX, Yu JT, Wang ND, Lu RC, Miao D, Tian Y, Tan L (2010) Blocking beta2-adrenergic receptor attenuates acute stress-induced amyloid beta peptides production. Brain Res 1317:305–310. doi:10.1016/j.brainres.2009.12.087
Zhang H, Liu J, Sun S, Pchitskaya E, Popugaeva E, Bezprozvanny I (2015) Calcium signaling, excitability, and synaptic plasticity defects in a mouse model of Alzheimer’s disease. J Alzheimers Dis 45:561–580. doi:10.3233/JAD-142427
Zhang H, Sun S, Herreman A, De Strooper B, Bezprozvanny I (2010) Role of presenilins in neuronal calcium homeostasis. J Neurosci 30:8566–8580. doi:10.1523/JNEUROSCI.1554-10.2010
Acknowledgements
This work was supported by NHLBI-R01HL061503 (ARM), NHLBI-R01HL102040 (ARM), NIAMS-R01AR060037 (ARM), NIH T32 HL120826 (ARM), NINDS-R25NS076445 (ARM), the Fondation Leducq and a generous donation from Carol Stix (ARM); Inserm, Philipp Foundation and Schaefer Award from Columbia University (AL); LECMA (Ligue Européenne Contre la Maladie d’Alzheimer) (MC); LABEX (Excellence Laboratory, Program Investment for the Future), DISTALZ (Development of Innovative Strategies for a Transdisciplinary approach to Alzheimer’s disease) (FC), the University Hospital Federation (FHU)-OncoAge (FC), the Fondation Pompidou (FC); NIA-RO1AG030205 (GES). The authors thank Steven Siegelbaum, Eric Kandel and Richard Axel for helpful discussions and comments on the paper and Valerie Scheuermann for preliminary biochemical experiments.
Author information
Authors and Affiliations
Contributions
ARM and MC independently conceived the study. XL, MC, FC, AL and ARM designed the studies, analyzed data and wrote the manuscript. XL conducted studies on APP +/− /PS1 +/−, RyR2-S2808D +/+, RyR2-S2808A +/+ and RyR2-S2808A +/+ × APP +/− /PS1 +/− mouse colonies. SR designed and conducted biochemistry assays of RyR2. MC, AL, RB, IL, CB and FD conducted studies on the 3 × Tg-AD mice. AM, NS and AL designed and conducted RyR2 single-channel analyses. AFT conducted the immunohistochemistry experiments on APP +/− /PS1 +/− and RyR2-S2808A +/+ × APP +/− /PS1 +/− mice and analyzed the data. CAB, SC and GES designed, conducted and performed patch-clamp Ca2+ imaging and sEPSP experiments and analyzed the data. OA analyzed the electrophysiological recordings and behavioral studies. MS designed the experiments, analyzed data and edited the manuscript.
Corresponding authors
Ethics declarations
Ethical approval
All applicable international, national and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
In vivo experiments were performed in accordance with the regulations of the Institutional Animal Care and Use Committee of Columbia University (APP+/−/PS1+/−, RyR2-S2808A +/+ , and RyR2-S2808D +/+ mice) and with the guidelines established by the European Community Council (Directive of November 24th, 1986) and approved by the Nice University Animal care and use Committee and the National Council on animal care of the Ministry of Health (Project No: NCE/2013-152) (3 × Tg-AD mice).
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical studies.
Informed consent
Informed consent for tissue donation for research was obtained by the brain banks under their approval procedures.
Conflict of interest
ARM is a board member and owns shares in ARMGO Pharma Inc., which is targeting RyR channels for therapeutic purposes.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lacampagne, A., Liu, X., Reiken, S. et al. Post-translational remodeling of ryanodine receptor induces calcium leak leading to Alzheimer’s disease-like pathologies and cognitive deficits. Acta Neuropathol 134, 749–767 (2017). https://doi.org/10.1007/s00401-017-1733-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00401-017-1733-7