Abstract
Resident cardiac macrophages (rcMacs) are integral components of the myocardium where they have key roles for tissue homeostasis and in response to inflammation, tissue injury and remodelling. In this review, we summarize the current knowledge and limitations associated with the rcMacs studies. We describe their specific role and contribution in various processes such as electrical conduction, efferocytosis, inflammation, tissue development, remodelling and regeneration in both the healthy and the disease state. We also outline research challenges and technical complications associated with rcMac research. Recent technological developments and contemporary immunological techniques are now offering new opportunities to investigate the separate contribution of rcMac in respect to recruited monocytes and other cardiac cells. Finally, we discuss new therapeutic strategies, such as drugs or non-coding RNAs, which can influence rcMac phenotype and their response to inflammation. These novel approaches will allow for a deeper understanding of this cardiac endogenous cell type and might lead to the development of more specific and effective therapeutic strategies to boost the heart’s intrinsic reparative capacity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
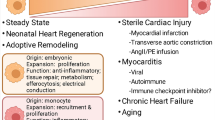
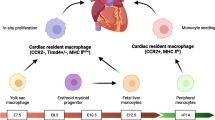
For several decades, bone marrow-derived macrophages were considered as the only large phagocytes involved in homeostasis, tissue healing, and defence against pathogens. Emerging evidence has overturned this dogma and has shown that resident macrophages (rMacs) are also fundamental players in a plethora of functions and cellular interactions both in homeostasis and in the modulation of the inflammatory response following injury and in tissue remodelling. Originating from the yolk sac or fetal liver progenitors [45], tissue rMacs inhabit various organs such as the bone marrow [58], lungs [76], liver [12], pancreas [17], brain [96], and heart [34]. Differently from circulating immune cells, rMac retain tissue-specific features. This population is made up of macrophages ontogenetically older than bone marrow-derived macrophages [95], they are evolutionarily conserved [30] and present throughout the lifetime. They can proliferate in situ and this process is exacerbated during inflammation [41]. In murine cardiac tissue, resident cardiac macrophages (rcMac) are reported to constitute up to 5–10% of the non-myocyte population, a percentage that increases dramatically following cardiac damage [50, 89]. With their peculiar spindle-like morphology, these resident immune cells take part in a large variety of physiological mechanisms which indeed include efferocytosis [26] but also immune surveillance, cardiac conduction [78]. These technologies have mostly been applied to murine models and they have identified different waves of rcMac formation [78]. Distinct lineages of rcMacs exist within the ventricular myocardium of the develo** heart and playing as essential regulators during cardiac development [64]. According to their cardiac localization and origin, it is possible to identify at least two distinct subsets of macrophages, CCR2− and CCR2+ (C–C chemokine receptor type 2) [64]. CCR2− cells originate from yolk sac progenitors, whereas CCR2+ derive from fetal monocyte progenitors, which is also reflected in their divergent gene expression profiles [64]. CCR2− cells are the first macrophage population appearing in the cardiac tissue at embryonic day 12.5 (E12.5), whereas CCR2+ inhabits the heart at E14.5. These cells are also confined in different regions of the heart [64]. More specifically, CCR2− are mostly found within the myocardial wall and in proximity to the coronary vasculature, whereas CCR2+ are in the trabecular projection of the endocardium [64]. These macrophages remain in the cardiac tissue for their entire life-span. For their embryonic origin and intrinsic self-renewal capacity, CCR2− rMacs are also defined as “resident population”. On the contrary, CCR2+ subset originates from haematopoiesis and their number is ensured by recruitment of circulating monocytes. For this reason, this subset is also defined as “non-resident population” [64]. Clinically, the association of CCR2+ macrophages abundance on LV remodeling and cardiac function has been shown in patient with heart failure [11].
During their development and in response to different environmental stimuli and functional responses, macrophages can be activated and functionally categorized into certain subgroups including M1, or M2 phenotypes. It is important to reiterate that this classification does not appropriately depict the in vivo spectrum of macrophage sub-populations present in both the healthy and diseased myocardium. In vitro this heterogeneity is reduced and the stimulation is applied in a more controlled environment, as such this simplified definition of M1/M2 is more acceptable. M1 or “classical” activated macrophages are pro-inflammatory phagocytic cells involved in the initial stages of inflammation and this phenotype is generally attributed by infiltrating monocytes [107]. Differently, the M2 or “alternative” activated macrophages are anti-inflammatory cells implicated in the resolution of the inflammatory process [88] and normally rcMacs in steady-state heart reflect this phenotype [107]. In vitro, M1 cells are known to secrete pro-inflammatory cytokines such as nitric oxide (NO), tumor necrosis factor (TNF-α), and interleukin 12p70 (IL-12p70) thus eliciting a robust inflammatory response [110]. On the contrary, the M2 in vitro activation leads to anti-inflammatory cytokines secretion which includes transforming growth factor (TGF-β), interleukin 10 (IL-10), and arginase-1 (Arg1). These cytokines support the repression of the inflammatory response, favour tissue healing and collagen deposition [74, 110]. The M1 or M2 phenotype is not permanent and can change. It was recently reported that rcMacs (mostly M2) can transition to M1-like phenotype in aged mice [69]. The M1/M2 paradigm was not only proposed based on the activation status, but it was also confirmed by distinct metabolic profiles, alterations in cell morphology [16], gene transcription [66] and functional efferocytosis [33, 37, 47, 54].
Another important aspect of macrophage biology is the heterogeneity in origin and phenotype following cardiac injury or during tissue remodelling. In this context, several markers are efficiently used to distinguish infiltrating and rcMacs, unfortunately, they are often not consistently expressed across animal species thus complicating the translation of research findings. Transgenic animals with fluorophore-labelled macrophages [33] or Cre-loxP macrophage reporter mice [99] can be helpful to provide informative data of specific cell types and overcome technical issues associated with antibody combinations. To date, one of the most common markers to discriminate resident and non-rMacs is CCR2−/+ which is conserved in human, rat, and mouse [10, 34]. Other options are C-X-3-C Motif Chemokine Receptor 1 (CX3CR1) and the major histocompatibility complex class II (MHCII). Using these markers, CX3CR1+MHCII− embryonic macrophages were identified in hearts from new-born mice and it was demonstrated how they tent to progressively diversify by increasing MHCII expression and decreasing CX3CR1 expression during aging [79]. In human, HLA-DR represents human homologue of MHC-II and human cardiac macrophages could be subdivided into three distinct subsets (CCR2+HLA-DRlow; CCR2+HLA-DRhigh; CCR−HLA-DRhigh) based on CCR2 and HLA-DR [11]. Alternatively, lymphocyte antigen 6 complex locus C (Ly6C) and MHCII were also used to efficiently distinguish four distinct subgroups of murine macrophages [39, 111]. Ly6C−/CCR2−/MHCIIhigh and MHCIIlow were shown to label macrophages deriving from the yolk sac, while Ly6C+CCR2− and Ly6C+CCR2+ are macrophages deriving from haematopoiesis [39, 111]. In rats, Ly6C marker is replaced by CD43high/low [1], whereas for human samples the equivalent marker is CD14 [11]. Recently, TIMD4 (T-cell immunoglobulin and mucin domain containing 4) and LYVE1 (Lymphatic vessel endothelial receptor 1) were identified as new markers for murine rcMacs [28].
Other common macrophage markers in human, mouse, and rat are CD68 [20, 29, 46, 112], MerTK (myeloid-epithelial-reproductive tyrosine kinase) [38], Mac-3 [70], galactose-specific lectin 3 (Galectin 3) [85] and CD163 [1, 29], these markers, however, do not discriminate between resident and non-rMacs. Other options are F4/80 [8, 105] which is mouse-specific and CD169 [29, 112] or CD64 [38] used for rat and mouse tissue [6, 98]. In human specimens, EMR1 (epidermal growth factor-like module-containing mucin-like hormone receptor-like 1) is the homolog of F4/80, and it labels both macrophages and granulocytes [4, 48]. CD11b (ITGAM) is also not sufficiently specific as it targets monocytes, neutrophils, and natural killer cells (NK cells) [70, 113]. A completely different set of markers is used to discriminate in vitro M1 and M2 macrophages. Inducible nitric oxidase (iNOS/NOS2) has been considered for several years a standard M1 marker [94]. Recently, the classic perception of CMs being the sole cellular units able to propagate the cardiac electrical impulse has been revisited. Indeed, it has been reported that the action potential propagation and CM contraction can be altered also by cell–cell interactions and communication between stromal cells and CMs [59]. Fibroblasts (FBs) play a key role in CM contraction in both physiological and pathological conditions [93]. Several in vitro studies have demonstrated the crucial role of direct contact between cardiac FBs and CMs to regulate electronic coupling [59, 86]. More recently, Hulsmans et al. were the first to demonstrate that rcMacs are also important mediators in this process and can alter electrical conduction [18]. These EV-YF1-enriched exosomes target macrophages leading to an increase in the production and secretion of IL-10 [18]. In a co-culture of CMs and macrophages, it was observed that the overexpression of EV-YF1 in macrophages, results in a cardioprotective outcome through IL-10 secretion [18]. In vivo, rats subjected to MI and treated with EV-YF1 displayed a reduction of the infarct area, highlighting the cardioprotective effect of this Y RNA fragment [18]. Similarly, exosomes secreted by CDCs can be enriched with different miRNA including miRNA-181b which were shown to induce macrophage polarization towards a cardioprotective phenotype with associated beneficial effect at a tissue level [24]. miR-155 is overexpressed in cardiac macrophages. In a mouse model of myocarditis, this specific microRNA was highly expressed by infiltrating macrophages [22]. The systemic knockdown of miR-155 leads to reduced infiltration of monocyte-derived macrophages and reduced cardiac damage [22]. In line with this, the pro-inflammatory role miR-155 has been confirmed also in a pressure-overload mouse model [84]. Mice with a deletion of miR-155 in macrophages show reduced hypertrophy and inflammation [84], suggesting its potential for therapeutic applications. Differently, lncRNA-Macrophages M2 polarization (MMP2P) is upregulated in M2, but not in M1 macrophages [19]. Moreover, the knockdown of lncRNA-MMP2P inhibits the polarization of macrophages towards the M2 phenotype by decreasing the phosphorylation of signal transducer and activator of transcription 6 (STAT6) [19].
Conclusions
Recent technological developments and contemporary immunological techniques are offering new opportunities to identify and study the roles and contribution of rcMac in respect to recruited monocytes and other cardiac cells. These novel approaches have already allowed scientist to better understand rcMac origin, phenotypic profile and their functional contribution in myocardial function. Basic and pre-clinical studies which involve the use of drugs or non-coding RNAs also demonstrated the potential of rcMac to regulate cellular interactions thus suggesting their use to modulate and potentially prevent tissue remodelling. The emerging evidence is also highlighting the detrimental effects induced by uncontrolled responses of this cell type. The future of macrophage-modulated therapy will have to take advantage of the mechanistic pathways that coordinate tissue repair and exploit them to develop more precise and effective therapeutic strategies.
References
Ahuja V, Miller SE, Howell DN (1995) Identification of two subpopulations of rat monocytes expressing disparate molecular forms and quantities of CD43. Cell Immunol 163:59–69. https://doi.org/10.1006/cimm.1995.1099
Alvarado-Vazquez PA, Bernal L, Paige CA, Grosick RL, Moracho Vilrriales C, Ferreira DW, Ulecia-Morón C, Romero-Sandoval EA (2017) Macrophage-specific nanotechnology-driven CD163 overexpression in human macrophages results in an M2 phenotype under inflammatory conditions. Immunobiology 222:900–912. https://doi.org/10.1016/j.imbio.2017.05.011
Van Amerongen MJ, Harmsen MC, Van Rooijen N, Petersen AH, Van Luyn MJA (2007) Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol 170:818–829. https://doi.org/10.2353/ajpath.2007.060547
Ancuta P, Liu KY, Misra V, Wacleche VS, Gosselin A, Zhou X, Gabuzda D (2009) Transcriptional profiling reveals developmental relationship and distinct biological functions of CD16+ and CD16− monocyte subsets. BMC Genomics 10:403. https://doi.org/10.1186/1471-2164-10-403
Aouadi M, Tesz GJ, Nicoloro SM, Wang M, Chouinard M, Soto E, Ostroff GR, Czech MP (2009) Orally delivered siRNA targeting macrophage Map4k4 suppresses systemic inflammation. Nature 458:1180–1184. https://doi.org/10.1038/nature07774
Asano K, Kikuchi K, Tanaka M (2018) CD169 macrophages regulate immune responses toward particulate materials in the circulating fluid. J Biochem 164:77–85. https://doi.org/10.1093/jb/mvy050
Aurora AB, Porrello ER, Tan W, Mahmoud AI, Hill JA, Bassel-Duby R, Sadek HA, Olson EN (2014) Macrophages are required for neonatal heart regeneration. J Clin Invest 124:1382–1392. https://doi.org/10.1172/JCI72181
Austyn JM, Gordon S (1981) F4/80, a monoclonal antibody directed specifically against the mouse macrophage. Eur J Immunol 11:805–815. https://doi.org/10.1002/eji.1830111013
Bagalkot V, Badgeley MA, Kampfrath T, Deiuliis JA, Rajagopalan S, Maiseyeu A (2015) Hybrid nanoparticles improve targeting to inflammatory macrophages through phagocytic signals. J Control Release 217:243–255. https://doi.org/10.1016/j.jconrel.2015.09.027
Bajpai G, Bredemeyer A, Li W, Zaitsev K, Koenig AL, Lokshina I, Mohan J, Ivey B, Hsiao HM, Weinheimer C, Kovacs A, Epelman S, Artyomov M, Kreisel D, Lavine KJ (2019) Tissue resident CCR2− and CCR2+ cardiac macrophages differentially orchestrate monocyte recruitment and fate specification following myocardial injury. Circ Res 124:263–278. https://doi.org/10.1161/CIRCRESAHA.118.314028
Bajpai G, Schneider C, Wong N, Bredemeyer A, Hulsmans M, Nahrendorf M, Epelman S, Kreisel D, Liu Y, Itoh A, Shankar TS, Selzman CH, Drakos SG, Lavine KJ (2018) The human heart contains distinct macrophage subsets with divergent origins and functions. Nat Med 24:1234–1245. https://doi.org/10.1038/s41591-018-0059-x
Beattie L, Sawtell A, Mann J, Frame TCM, Teal B, de Labastida RF, Brown N, Walwyn-Brown K, Moore JWJ, MacDonald S, Lim EK, Dalton JE, Engwerda CR, MacDonald KP, Kaye PM (2016) Bone marrow-derived and resident liver macrophages display unique transcriptomic signatures but similar biological functions. J Hepatol 65:758–768. https://doi.org/10.1016/j.jhep.2016.05.037
Bejerano T, Etzion S, Elyagon S, Etzion Y, Cohen S (2018) Nanoparticle delivery of miRNA-21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett 18:5885–5891. https://doi.org/10.1021/acs.nanolett.8b02578
Ben-Mordechai T, Holbova R, Landa-Rouben N, Harel-Adar T, Feinberg MS, Abd Elrahman I, Blum G, Epstein FH, Silman Z, Cohen S, Leor J (2013) Macrophage subpopulations are essential for infarct repair with and without stem cell therapy. J Am Coll Cardiol 62:1890–1901. https://doi.org/10.1016/j.jacc.2013.07.057
Ben-Mordechai T, Palevski D, Glucksam-Galnoy Y, Elron-Gross I, Margalit R, Leor J (2015) Targeting macrophage subsets for infarct repair. J Cardiovasc Pharmacol Ther 20:36–51. https://doi.org/10.1177/1074248414534916
Bertani FR, Mozetic P, Fioramonti M, Iuliani M, Ribelli G, Pantano F, Santini D, Tonini G, Trombetta M, Businaro L, Selci S, Rainer A (2017) Classification of M1/M2-polarized human macrophages by label-free hyperspectral reflectance confocal microscopy and multivariate analysis. Sci Rep 7:8965. https://doi.org/10.1038/s41598-017-08121-8
Calderon B, Carrero JA, Ferris ST, Sojka DK, Moore L, Epelman S, Murphy KM, Yokoyama WM, Randolph GJ, Unanue ER (2015) The pancreas anatomy conditions the origin and properties of resident macrophages. J Exp Med 212:1497–1512. https://doi.org/10.1084/jem.20150496
Cambier L, Couto G, Ibrahim A, Echavez AK, Valle J, Liu W, Kreke M, Smith RR, Marbán L, Marbán E (2017) Y RNA fragment in extracellular vesicles confers cardioprotection via modulation of IL-10 expression and secretion. EMBO Mol Med 9:337–352. https://doi.org/10.15252/emmm.201606924
Cao J, Dong R, Jiang L, Gong Y, Yuan M, You J, Meng W, Chen Z, Zhang N, Weng Q, Zhu H, He Q, Ying M, Yang B (2019) LncRNA-Mm2p identified as a modulator of macrophage M2 polarization. Cancer Immunol Res 7:292–305. https://doi.org/10.1158/2326-6066.CIR-18-0145
Chistiakov DA, Killingsworth MC, Myasoedova VA, Orekhov AN, Bobryshev YV (2017) CD68/macrosialin: not just a histochemical marker. Lab Investig 97:4–13. https://doi.org/10.1038/labinvest.2016.116
Corliss BA, Azimi MS, Munson JM, Peirce SM, Murfee WL (2016) Macrophages: an inflammatory link between angiogenesis and lymphangiogenesis. Microcirculation 23:95–121. https://doi.org/10.1111/micc.12259
Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, Summer G, Coort SLM, Hazebroek M, Van Leeuwen R, Gijbels MJJ, Wijnands E, Biessen EAL, De Winther MPJ, Stassen FRM, Carmeliet P, Kauppinen S, Schroen B, Heymans S (2012) MicroRNA profiling identifies MicroRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral MyoCarditis. Circ Res 111:415–425. https://doi.org/10.1161/CIRCRESAHA.112.267443
Courties G, Heidt T, Sebas M, Iwamoto Y, Jeon D, Truelove J, Tricot B, Wojtkiewicz G, Dutta P, Sager HB, Borodovsky A, Novobrantseva T, Klebanov B, Fitzgerald K, Anderson DG, Libby P, Swirski FK, Weissleder R, Nahrendorf M (2014) In vivo silencing of the transcription factor IRF5 reprograms the macrophage phenotype and improves infarct healing. J Am Coll Cardiol 63:1556–1566. https://doi.org/10.1016/j.jacc.2013.11.023
De Couto G, Gallet R, Cambier L, Jaghatspanyan E, Makkar N, Dawkins JF, Berman BP, Marbán E (2017) Exosomal microRNA transfer into macrophages mediates cellular postconditioning. Circulation 136:200–214. https://doi.org/10.1161/CIRCULATIONAHA.116.024590
De Couto G, Liu W, Tseliou E, Sun B, Makkar N, Kanazawa H, Arditi M, Marbán E (2015) Macrophages mediate cardioprotective cellular postconditioning in acute myocardial infarction. J Clin Invest 125:3147–3162. https://doi.org/10.1172/JCI81321
DeBerge M, Yeap XY, Dehn S, Zhang S, Grigoryeva L, Misener S, Procissi D, Zhou X, Lee DC, Muller WA, Luo X, Rothlin C, Tabas I, Thorp EB (2017) MerTK cleavage on resident cardiac macrophages compromises repair after myocardial ischemia reperfusion injury. Circ Res 121:930–940. https://doi.org/10.1161/CIRCRESAHA.117.311327
Dehn S, Thorp EB (2018) Myeloid receptor CD36 is required for early phagocytosis of myocardial infarcts and induction of Nr4a1-dependent mechanisms of cardiac repair. FASEB J 32:254–264. https://doi.org/10.1096/fj.201700450R
Dick SA, Macklin JA, Nejat S, Momen A, Clemente-Casares X, Althagafi MG, Chen J, Kantores C, Hosseinzadeh S, Aronoff L, Wong A, Zaman R, Barbu I, Besla R, Lavine KJ, Razani B, Ginhoux F, Husain M, Cybulsky MI, Robbins CS, Epelman S (2019) Self-renewing resident cardiac macrophages limit adverse remodeling following myocardial infarction. Nat Immunol 20:29–39. https://doi.org/10.1038/s41590-018-0272-2
Dijkstra CD, Döpp EA, Joling P, Kraal G (1985) The heterogeneity of mononuclear phagocytes in lymphoid organs: distinct macrophage subpopulations in rat recognized by monoclonal antibodies ED1, ED2 and ED3. In: Klaus GGB (ed) Microenvironemnts in the lymphoid system. Springer, Boston, pp 409–419. https://doi.org/10.1007/978-1-4613-2463-8_50
Edholm E, Rhoo KH, Robert J (2017) Evolutionary aspects of macrophages. Macrophages Orig Funct Biointervention 63:3–22. https://doi.org/10.1007/978-3-319-54090-0
Elamm C, Fairweather DL, Cooper LT (2012) Pathogenesis and diagnosis of myocarditis. Heart 98:835–840. https://doi.org/10.1136/heartjnl-2012-301686
Eligini S, Cosentino N, Fiorelli S, Fabbiocchi F, Niccoli G, Refaat H, Camera M, Calligaris G, De Martini S, Bonomi A, Veglia F, Fracassi F, Crea F, Marenzi G, Tremoli E (2019) Biological profile of monocyte-derived macrophages in coronary heart disease patients: implications for plaque morphology. Sci Rep 9:8680. https://doi.org/10.1038/s41598-019-44847-3
Ellett F, Pase L, Hayman JW, Andrianopoulos A, Lieschke GJ (2011) mpeg1 promoter transgenes direct macrophage-lineage expression in zebrafish. Blood 117:e49-56. https://doi.org/10.1182/blood-2010-10-314120
Epelman S, Lavine KJ, Beaudin AE, Sojka DK, Carrero JA, Calderon B, Brija T, Gautier EL, Ivanov S, Satpathy AT, Schilling JD, Schwendener R, Sergin I, Razani B, Forsberg EC, Yokoyama WM, Unanue ER, Colonna M, Randolph GJ, Mann DL (2014) Embryonic and adult-derived resident cardiac macrophages are maintained through distinct mechanisms at steady state and during inflammation. Immunity 40:91–104. https://doi.org/10.1016/j.immuni.2013.11.019
Ferraro B, Leoni G, Hinkel R, Ormanns S, Paulin N, Ortega-Gomez A, Viola JR, de Jong R, Bongiovanni D, Bozoglu T, Maas SL, D’Amico M, Kessler T, Zeller T, Hristov M, Reutelingsperger C, Sager HB, Döring Y, Nahrendorf M, Kupatt C, Soehnlein O (2019) Pro-angiogenic macrophage phenotype to promote myocardial repair. J Am Coll Cardiol 73:2990–3002. https://doi.org/10.1016/j.jacc.2019.03.503
Flores AM, Hosseini-Nassab N, Jarr KU, Ye J, Zhu X, Wirka R, Koh AL, Tsantilas P, Wang Y, Nanda V, Kojima Y, Zeng Y, Lotfi M, Sinclair R, Weissman IL, Ingelsson E, Smith BR, Leeper NJ (2020) Pro-efferocytic nanoparticles are specifically taken up by lesional macrophages and prevent atherosclerosis. Nat Nanotechnol 15:154–161. https://doi.org/10.1038/s41565-019-0619-3
de Fulco TO, Andrade PR, de Barbosa MGM, Pinto TGT, Ferreira PF, Ferreira H, da Nery JAC, Real SC, Borges VM, Moraes MO, Sarno EN, Sampaio EP, Pinheiro RO (2014) Effect of apoptotic cell recognition on macrophage polarization and mycobacterial persistence. Infect Immun 82:3968–3978. https://doi.org/10.1128/IAI.02194-14
Gautiar EL, Shay T, Miller J, Greter M, Jakubzick C, Ivanov S, Helft J, Chow A, Elpek KG, Gordonov S, Mazloom AR, Ma’Ayan A, Chua WJ, Hansen TH, Turley SJ, Merad M, Randolph GJ, Best AJ, Knell J, Goldrath A, Brown B, Jojic V, Koller D, Cohen N, Brenner M, Regev A, Fletcher A, Bellemare-Pelletier A, Malhotra D, Jianu R, Laidlaw D, Collins J, Narayan K, Sylvia K, Kang J, Gazit R, Garrison BS, Rossi DJ, Kim F, Rao TN, Wagers A, Shinton SA, Hardy RR, Monach P, Bezman NA, Sun JC, Kim CC, Lanier LL, Heng T, Kreslavsky T, Painter M, Ericson J, Davis S, Mathis D, Benoist C (2012) Gene-expression profiles and transcriptional regulatory pathways that underlie the identity and diversity of mouse tissue macrophages. Nat Immunol. 13:1118–1128. https://doi.org/10.1038/ni.2419
Geissmann F, Jung S, Littman DR (2003) Blood monocytes consist of two principal subsets with distinct migratory properties. Immunity 19:71–82. https://doi.org/10.1016/S1074-7613(03)00174-2
Getts DR, Terry RL, Getts MT, Deffrasnes C, Müller M, Vreden C, Ashhurst TM, Chami B, McCarthy D, Wu H, ** M, Martin A, Shae LD, Witting P, Kansas GS, Kühn J, Hafezi W, Campbell IL, Reilly D, Say J, Brown L, White MY, Cordwell SJ, Chadban SJ, Thorp EB, Bao S, Miller SD, King NJC (2014) Therapeutic inflammatory monocyte modulation using immune-modifying microparticles. Sci Transl Med 6:219ra7. https://doi.org/10.1126/scitranslmed.3007563
Ginhoux F, Jung S (2014) Monocytes and macrophages: Developmental pathways and tissue homeostasis. Nat Rev Immunol 14:392–404. https://doi.org/10.1038/nri3671
De Giusti CJ, Ure AE, Rivadeneyra L, Schattner M, Gomez RM (2015) Macrophages and galectin 3 play critical roles in CVB3-induced murine acute myocarditis and chronic fibrosis. J Mol Cell Cardiol 85:58–70. https://doi.org/10.1016/j.yjmcc.2015.05.010
Godwin JW, Debuque R, Salimova E, Rosenthal NA (2017) Heart regeneration in the salamander relies on macrophage-mediated control of fibroblast activation and the extracellular landscape. NPJ Regen Med 2:22. https://doi.org/10.1038/s41536-017-0027-y
Godwin JW, Pinto AR, Rosenthal NA (2013) Macrophages are required for adult salamander limb regeneration. Proc Natl Acad Sci USA 110:9415–9420. https://doi.org/10.1073/pnas.1300290110
Gomez Perdiguero E, Klapproth K, Schulz C, Busch K, Azzoni E, Crozet L, Garner H, Trouillet C, De Bruijn MF, Geissmann F, Rodewald HR (2015) Tissue-resident macrophages originate from yolk-sac-derived erythro-myeloid progenitors. Nature 518:547–551. https://doi.org/10.1038/nature13989
Gottfried E, Kunz-Schughart LA, Weber A, Rehli M, Peuker A, Müller A, Kastenberger M, Brockhoff G, Andreesen R, Kreutz M (2008) Expression of CD68 in non-myeloid cell types. Scand J Immunol 67:453–463. https://doi.org/10.1111/j.1365-3083.2008.02091.x
Haloul M, Oliveira ERA, Kader M, Wells JZ, Tominello TR, El Andaloussi A, Yates CC, Ismail N (2019) mTORC1-mediated polarization of M1 macrophages and their accumulation in the liver correlate with immunopathology in fatal ehrlichiosis. Sci Rep 9:14050. https://doi.org/10.1038/s41598-019-50320-y
Hamann J, Koning N, Pouwels W, Ulfman LH, van Eijk M, Stacey M, Lin HH, Gordon S, Kwakkenbos MJ (2007) EMR1, the human homolog of F4/80, is an eosinophil-specific receptor. Eur J Immunol 37:2797–2802. https://doi.org/10.1002/eji.200737553
Harel-Adar T, Ben MT, Amsalem Y, Feinberg MS, Leor J, Cohen S (2011) Modulation of cardiac macrophages by phosphatidylserine-presenting liposomes improves infarct repair. Proc Natl Acad Sci USA 108:1827–1832. https://doi.org/10.1073/pnas.1015623108
Heidt T, Courties G, Dutta P, Sager HB, Sebas M, Iwamoto Y, Sun Y, Da Silva N, Panizzi P, Van Der Lahn AM, Swirski FK, Weissleder R, Nahrendorf M (2014) Differential contribution of monocytes to heart macrophages in steady-state and after myocardial infarction. Circ Res 115:284–295. https://doi.org/10.1161/CIRCRESAHA.115.303567
Hitscherich PG, **e LH, Del Re D, Lee EJ (2019) The effects of macrophages on cardiomyocyte calcium-handling function using in vitro culture models. Physiol Rep 7:e14137. https://doi.org/10.14814/phy2.14137
Hoyer FF, Naxerova K, Schloss MJ, Hulsmans M, Nair AV, Dutta P, Calcagno DM, Herisson F, Anzai A, Sun Y, Wojtkiewicz G, Rohde D, Frodermann V, Vandoorne K, Courties G, Iwamoto Y, Garris CS, Williams DL, Breton S, Brown D, Whalen M, Libby P, Pittet MJ, King KR, Weissleder R, Swirski FK, Nahrendorf M (2019) Tissue-specific macrophage responses to remote injury impact the outcome of subsequent local immune challenge. Immunity 51:899-914.e.7. https://doi.org/10.1016/j.immuni.2019.10.010
Hulsmans M, Clauss S, **ao L, Aguirre AD, King KR, Hanley A, Hucker WJ, Wülfers EM, Seemann G, Courties G, Iwamoto Y, Sun Y, Savol AJ, Sager HB, Lavine KJ, Fishbein GA, Capen DE, Da Silva N, Miquerol L, Wakimoto H, Seidman CE, Seidman JG, Sadreyev RI, Naxerova K, Mitchell RN, Brown D, Libby P, Weissleder R, Swirski FK, Kohl P, Vinegoni C, Milan DJ, Ellinor PT, Nahrendorf M (2017) Macrophages facilitate electrical conduction in the heart. Cell 169:510–522. https://doi.org/10.1016/j.cell.2017.03.050
Igarashi Y, Nawaz A, Kado T, Bilal M, Kuwano T, Yamamoto S, Sasahara M, Jiuxiang X, Inujima A, Koizumi K, Imura J, Shibahara N, Usui I, Fujisaka S, Tobe K (2018) Partial depletion of CD206-positive M2-like macrophages induces proliferation of beige progenitors and enhances browning after cold stimulation. Sci Rep 8:14567. https://doi.org/10.1038/s41598-018-32803-6
Jablonski KA, Amici SA, Webb LM, Ruiz-Rosado JDD, Popovich PG, Partida-Sanchez S, Guerau-De-arellano M (2015) Novel markers to delineate murine M1 and M2 macrophages. PLoS ONE 8:14567. https://doi.org/10.1371/journal.pone.0145342
Jafarzadeh A, Chauhan P, Saha B, Jafarzadeh S, Nemati M (2020) Contribution of monocytes and macrophages to the local tissue inflammation and cytokine storm in COVID-19: Lessons from SARS and MERS, and potential therapeutic interventions. Life Sci 257:118102. https://doi.org/10.1016/j.lfs.2020.118102
Jain S, Tran TH, Amiji M (2015) Macrophage repolarization with targeted alginate nanoparticles containing IL-10 plasmid DNA for the treatment of experimental arthritis. Biomaterials 61:162–177. https://doi.org/10.1016/j.biomaterials.2015.05.028
Kaur S, Raggatt LJ, Batoon L, Hume DA, Levesque JP, Pettit AR (2017) Role of bone marrow macrophages in controlling homeostasis and repair in bone and bone marrow niches. Semin Cell Dev Biol 6:12–21. https://doi.org/10.1016/j.semcdb.2016.08.009
Kohl P, Gourdie RG (2014) Fibroblast-myocyte electrotonic coupling: Does it occur in native cardiac tissue? J Mol Cell Cardiol 70:37–46. https://doi.org/10.1016/j.yjmcc.2013.12.024
Korolnek T, Hamza I (2015) Macrophages and iron trafficking at the birth and death of red cells. Blood 125:2893–2897. https://doi.org/10.1182/blood-2014-12-567776
Kuballa P, Nolte WM, Castoreno AB, Xavier RJ (2012) Autophagy and the immune system. Annu Rev Immunol 30:611–646. https://doi.org/10.1146/annurev-immunol-020711-074948
Lavine KJ, Epelman S, Uchida K, Weber KJ, Nichols CG, Schilling JD, Ornitz DM, Randolph GJ, Mann DL (2016) Erratum: Distinct macrophage lineages contribute to disparate patterns of cardiac recovery and remodeling in the neonatal and adult heart (Proceedings of the National Academy of Sciences of the United States of America (2014) 111 (16029–16034) DOI 10.1073). Proc Natl Acad Sci USA 113:E1414
Lavine KJ, Pinto AR, Epelman S, Kopecky BJ, Clemente-Casares X, Godwin J, Rosenthal N, Kovacic JC (2018) The Macrophage in cardiac homeostasis and disease: JACC macrophage in CVD series (Part 4). J Am Coll Cardiol 72:2213–2230. https://doi.org/10.1016/j.jacc.2018.08.2149
Leid J, Carrelha J, Boukarabila H, Epelman S, Jacobsen SEW, Lavine KJ (2016) Primitive embryonic macrophages are required for coronary development and maturation. Circ Res 118:1498–1511. https://doi.org/10.1161/CIRCRESAHA.115.308270
Leuschner F, Rauch PJ, Ueno T, Gorbatov R, Marinelli B, Lee WW, Dutta P, Wei Y, Robbins C, Iwamoto Y, Sena B, Chudnovskiy A, Panizzi P, Keliher E, Higgins JM, Libby P, Moskowitz MA, Pittet MJ, Swirski FK, Weissleder R, Nahrendorf M (2012) Rapid monocyte kinetics in acute myocardial infarction are sustained by extramedullary monocytopoiesis. J Exp Med 209:123–137. https://doi.org/10.1084/jem.20111009
Li C, Menoret A, Farragher C, Ouyang Z, Bonin C, Holvoet P, Vella AT, Zhou B (2019) Single-cell transcriptomics-based MacSpectrum reveals macrophage activation signatures in diseases. JCI Insight 5:e126453. https://doi.org/10.1172/jci.insight.126453
Liu B, Zhang HG, Zhu Y, Jiang YH, Luo GP, Tang FQ, Jian Z, Bin XY (2017) Cardiac resident macrophages are involved in hypoxia-induced postnatal cardiomyocyte proliferation. Mol Med Rep 15:3541–3548. https://doi.org/10.3892/mmr.2017.6432
Ma F, Li Y, Jia L, Han Y, Cheng J, Li H, Qi Y, Du J (2012) Macrophage-stimulated cardiac fibroblast production of IL-6 is essential for TGF β/Smad activation and cardiac fibrosis induced by angiotensin II. PLoS ONE 7:e35144. https://doi.org/10.1371/journal.pone.0035144
Ma Y, Chiao YA, Clark R, Flynn ER, Yabluchanskiy A, Ghasemi O, Zouein F, Lindsey ML, ** YF (2015) Deriving a cardiac ageing signature to reveal MMP-9-dependent inflammatory signalling in senescence. Cardiovasc Res 106:421–431. https://doi.org/10.1093/cvr/cvv128
Ma Y, Mouton AJ, Lindsey ML (2018) Cardiac macrophage biology in the steady-state heart, the aging heart, and following myocardial infarction. Transl Res 191:15–28. https://doi.org/10.1016/j.trsl.2017.10.001
Ma Z, Zhang J, Xu X, Qu Y, Dong H, Dang J, Huo Z, Xu G (2019) LncRNA expression profile during autophagy and Malat1 function in macrophages. PLoS ONE 14:e0221104. https://doi.org/10.1371/journal.pone.0221104
MacHnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, MacHura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, Van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J (2009) Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15:545–552. https://doi.org/10.1038/nm.1960
Mahfoud F, Grtner B, Kindermann M, Ukena C, Gadomski K, Klingel K, Kandolf R, Bhm M, Kindermann I (2011) Virus serology in patients with suspected myocarditis: utility or futility? Eur Heart J 32:897–903. https://doi.org/10.1093/eurheartj/ehq493
Martinez FO, Gordon S, Locati M, Mantovani A (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177:7303–7311. https://doi.org/10.4049/jimmunol.177.10.7303
Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13:453–461. https://doi.org/10.2741/2692
Mathie SA, Dixon KL, Walker SA, Tyrrell V, Mondhe M, O’Donnell VB, Gregory LG, Lloyd CM (2015) Alveolar macrophages are sentinels of murine pulmonary homeostasis following inhaled antigen challenge. Allergy Eur J Allergy Clin Immunol 70:80–89. https://doi.org/10.1111/all.12536
McCartney SA, Vermi W, Lonardi S, Rossini C, Otero K, Calderon B, Gilfillan S, Diamond MS, Unanue ER, Colonna M (2011) RNA sensor-induced type I IFN prevents diabetes caused by a β cell-tropic virus in mice. J Clin Invest 121:1497–1507. https://doi.org/10.1172/JCI44005
McGrath KE, Frame JM, Palis J (2015) Early hematopoiesis and macrophage development. Semin Immunol 27:379–387. https://doi.org/10.1016/j.smim.2016.03.013
Molawi K, Wolf Y, Kandalla PK, Favret J, Hagemeyer N, Frenzel K, Pinto AR, Klapproth K, Henri S, Malissen B, Rodewald HR, Rosenthal NA, Bajenoff M, Prinz M, Jung S, Sieweke MH (2014) Progressive replacement of embryo-derived cardiac macrophages with age. J Exp Med 211:2151–2158. https://doi.org/10.1084/jem.20140639
Monnerat G, Alarcón ML, Vasconcellos LR, Hochman-Mendez C, Brasil G, Bassani RA, Casis O, Malan D, Travassos LH, Sepúlveda M, Burgos JI, Vila-Petroff M, Dutra FF, Bozza MT, Paiva CN, Carvalho AB, Bonomo A, Fleischmann BK, De Carvalho ACC, Medei E (2016) Macrophage-dependent IL-1β production induces cardiac arrhythmias in diabetic mice. Nat Commun 7:13344. https://doi.org/10.1038/ncomms13344
Nahrendorf M, Swirski FK (2013) Monocyte and macrophage heterogeneity in the heart. Circ Res 112:1624–1633. https://doi.org/10.1038/nri1733
Nicolás-Ávila JA, Lechuga-Vieco AV, Esteban-Martínez L, Sánchez-Díaz M, Díaz-García E, Santiago DJ, Rubio-Ponce A, Li JL, Balachander A, Quintana JA, Martínez-de-Mena R, Castejón-Vega B, Pun-García A, Través PG, Bonzón-Kulichenko E, García-Marqués F, Cussó L, A-González N, González-Guerra A, Roche-Molina M, Martin-Salamanca S, Crainiciuc G, Guzmán G, Larrazabal J, Herrero-Galán E, Alegre-Cebollada J, Lemke G, Rothlin CV, Jimenez-Borreguero LJ, Reyes G, Castrillo A, Desco M, Muñoz-Cánoves P, Ibáñez B, Torres M, Ng LG, Priori SG, Bueno H, Vázquez J, Cordero MD, Bernal JA, Enríquez JA, Hidalgo A (2020) A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183:94-109.e23. https://doi.org/10.1016/j.cell.2020.08.031
Nozaki N, Shishido T, Takeishi Y, Kubota I (2004) Modulation of doxorubicin-induced cardiac dysfunction in toll-like receptor-2-knockout mice. Circulation 110:2869–2874. https://doi.org/10.1161/01.CIR.0000146889.46519.27
O’Connell RM, Kahn D, Gibson WSJ, Round JL, Scholz RL, Chaudhuri AA, Kahn ME, Rao DS, Baltimore D (2010) MicroRNA-155 promotes autoimmune inflammation by enhancing inflammatory T cell development. Immunity 33:607–619. https://doi.org/10.1016/j.immuni.2010.09.009
de Oliveira FL, Gatto M, Bassi N, Luisetto R, Ghirardello A, Punzi L, Doria A (2015) Galectin-3 in autoimmunity and autoimmune diseases. Exp Biol Med 240:1019–1028. https://doi.org/10.1177/1535370215593826
Ongstad E, Kohl P (2016) Fibroblast-myocyte coupling in the heart: Potential relevance for therapeutic interventions. J Mol Cell Cardiol 91:238–246. https://doi.org/10.1016/j.yjmcc.2016.01.010
Peet C, Ivetic A, Bromage DI, Shah AM (2020) Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res 116:1101–1112. https://doi.org/10.1093/cvr/cvz336
Pesce JT, Ramalingam TR, Mentink-Kane MM, Wilson MS, Kasmi KCE, Smith AM, Thompson RW, Cheever AW, Murray PJ, Wynn TA (2009) Arginase-1-expressing macrophages suppress Th2 cytokine-driven inflammation and fibrosis. PLoS Pathog 5(5):e1000371. https://doi.org/10.1371/journal.ppat.1000371
Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’antoni ML, Debuque R, Chandran A, Wang L, Arora K, Rosenthal NA, Tallquist MD (2016) Revisiting cardiac cellular composition. Circ Res 118:400–409. https://doi.org/10.1161/CIRCRESAHA.115.307778
Porrello ER, Mahmoud AI, Simpson E, Hill JA, Richardson JA, Olson EN, Sadek HA (2011) Transient regenerative potential of the neonatal mouse heart. Science (80-) 331:1078–1080. https://doi.org/10.1126/science.1200708
Porrello ER, Mahmoud AI, Simpson E, Johnson BA, Grinsfelder D, Canseco D, Mammen PP, Rothermel BA, Olson EN, Sadek HA (2013) Regulation of neonatal and adult mammalian heart regeneration by the miR-15 family. Proc Natl Acad Sci USA 110:187–192. https://doi.org/10.1073/pnas.1208863110
Riad A, Bien S, Gratz M, Escher F, Westermann D, Heimesaat MM, Bereswill S, Krieg T, Felix SB, Schultheiss HP, Kroemer HK, Tschöpe C (2008) Toll-like receptor-4 deficiency attenuates doxorubicin-induced cardiomyopathy in mice. Eur J Heart Fail 10:233–243. https://doi.org/10.1016/j.ejheart.2008.01.004
Rohr S (2012) Arrhythmogenic implications of fibroblast-myocyte interactions. Circ Arrhythmia Electrophysiol 5:442–452. https://doi.org/10.1161/CIRCEP.110.957647
Rosenthal N (2017) A guardian of the heartbeat. N Engl J Med 377:84–86. https://doi.org/10.1056/NEJMcibr1705327
Schulz C, Perdiguero EG, Chorro L, Szabo-Rogers H, Cagnard N, Kierdorf K, Prinz M, Wu B, Jacobsen SEW, Pollard JW, Frampton J, Liu KJ, Geissmann F (2012) A lineage of myeloid cells independent of myb and hematopoietic stem cells. Science (80-) 336:86–90. https://doi.org/10.1126/science.1219179
Sevenich L (2018) Brain-resident microglia and blood-borne macrophages orchestrate central nervous system inflammation in neurodegenerative disorders and brain cancer. Front Immunol 9:697. https://doi.org/10.3389/fimmu.2018.00697
Shahid F, Lip GYH, Shantsila E (2018) Role of monocytes in heart failure and atrial fibrillation. J Am Heart Assoc 7:e007849. https://doi.org/10.1161/JAHA.117.0078497
Sheng J, Chen Q, Soncin I, Ng SL, Karjalainen K, Ruedl C (2017) A discrete subset of monocyte-derived cells among typical conventional type 2 dendritic cells can efficiently cross-present. Cell Rep 21:1203–1214. https://doi.org/10.1016/j.celrep.2017.10.024
Shi J, Hua L, Harmer D, Li P, Ren G (2018) Cre driver mice targeting macrophages. Methods Mol Biol 1784:263–275. https://doi.org/10.1007/978-1-4939-7837-3_24
Shiraishi M, Shintani Y, Shintani Y, Ishida H, Saba R, Yamaguchi A, Adachi H, Yashiro K, Suzuki K (2016) Alternatively activated macrophages determine repair of the infarcted adult murine heart. J Clin Invest 126:2151–2166. https://doi.org/10.1172/JCI85782
Shirakawa K, Endo J, Kataoka M, Katsumata Y, Anzai A, Moriyama H, Kitakata H, Hiraide T, Ko S, Goto S, Ichihara G, Fukuda K, Minamino T, Sano M (2020) MerTK expression and ERK activation are essential for the functional maturation of osteopontin-producing reparative macrophages after myocardial infarction. J Am Heart Assoc 9:e017071. https://doi.org/10.1161/JAHA.120.017071
Simões FC, Cahill TJ, Kenyon A, Gavriouchkina D, Vieira JM, Sun X, Pezzolla D, Ravaud C, Masmanian E, Weinberger M, Mayes S, Lemieux ME, Barnette DN, Gunadasa-Rohling M, Williams RM, Greaves DR, Trinh LA, Fraser SE, Dallas SL, Choudhury RP, Sauka-Spengler T, Riley PR (2020) Macrophages directly contribute collagen to scar formation during zebrafish heart regeneration and mouse heart repair. Nat Commun 11:600. https://doi.org/10.1038/s41467-019-14263-2
Spiller KL, Anfang RR, Spiller KJ, Ng J, Nakazawa KR, Daulton JW, Vunjak-Novakovic G (2014) The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 35:4477–4488. https://doi.org/10.1016/j.biomaterials.2014.02.012
Vagnozzi RJ, Maillet M, Sargent MA, Khalil H, Johansen AKZ, Schwanekamp JA, York AJ, Huang V, Nahrendorf M, Sadayappan S, Molkentin JD (2020) An acute immune response underlies the benefit of cardiac stem cell therapy. Nature 577:405–409. https://doi.org/10.1038/s41586-019-1802-2
Waddell LA, Lefevre L, Bush SJ, Raper A, Young R, Lisowski ZM, McCulloch MEB, Muriuki C, Sauter KA, Clark EL, Irvine KM, Pridans C, Hope JC, Hume DA (2018) ADGRE1 (EMR1, F4/80) is a rapidly-evolving gene expressed in mammalian monocyte-macrophages. Front Immunol 9:2246. https://doi.org/10.3389/fimmu.2018.02246
Wang Z, Cui M, Shah AM, Ye W, Tan W, Min YL, Botten GA, Shelton JM, Liu N, Bassel-Duby R, Olson EN (2019) Mechanistic basis of neonatal heart regeneration revealed by transcriptome and histone modification profiling. Proc Natl Acad Sci USA 116:18455–18465. https://doi.org/10.1073/pnas.1905824116
Wang Z, Koenig AL, Lavine KJ, Apte RS (2019) Macrophage plasticity and function in the eye and heart. Trends Immunol 40:825–841. https://doi.org/10.1016/j.it.2019.07.002
Xue Q, Yan Y, Zhang R, **ong H (2018) Regulation of iNOS on immune cells and its role in diseases. Int J Mol Sci 19:3805. https://doi.org/10.3390/ijms19123805
Yang Z, Ming XF (2014) Functions of arginase isoforms in macrophage inflammatory responses: Impact on cardiovascular diseases and metabolic disorders. Front Immunol 5:533. https://doi.org/10.3389/fimmu.2014.00533
Yap J, Cabrera-Fuentes HA, Irei J, Hausenloy DJ, Boisvert WA (2019) Role of macrophages in cardioprotection. Int J Mol Sci 20:2474. https://doi.org/10.3390/ijms20102474
Yona S, Kim KW, Wolf Y, Mildner A, Varol D, Breker M, Strauss-Ayali D, Viukov S, Guilliams M, Misharin A, Hume DA, Perlman H, Malissen B, Zelzer E, Jung S (2013) Fate map** reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity 38:79–91. https://doi.org/10.1016/j.immuni.2012.12.001
Yoon S, Yoo HJ, Shim NR, Baek SY, Kim BS, Kim JB, Jun EJ, Son YK, Lee SY, Yoo YH (2003) Immunohistochemical characterization of macrophage and dendritic cell subpopulations of the spleen, thymus, tongue and heart in cyclophosphamide-induced immunosuppressed rat. J Vet Med Ser C Anat Histol Embryol 32:80–88. https://doi.org/10.1046/j.1439-0264.2003.00454.x
Zhou H, Liao J, Aloor J, Nie H, Wilson BC, Fessler MB, Gao H-M, Hong J-S (2013) CD11b/CD18 (Mac-1) is a novel surface receptor for extracellular double-stranded rna to mediate cellular inflammatory responses. J Immunol 190:115–125. https://doi.org/10.4049/jimmunol.1202136
Acknowledgements
Prof. Thum is supported by Grants from ERC Longheart and ERANET CVD Expert and DFG (KFO311).
Funding
Open Access funding enabled and organized by Projekt DEAL.. TT is founder and shareholder of Cardior Pharmaceuticals GmbH. TT has filed and licensed patents in the field of noncoding RNAs. TT received funding/support from Amicus Therapeutics, Boehringer Ingelheim, Novo Nordisk, Sanofi-Genzyme, Takeda (all outside of the here presented work).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sansonetti, M., Waleczek, F.J.G., Jung, M. et al. Resident cardiac macrophages: crucial modulators of cardiac (patho)physiology. Basic Res Cardiol 115, 77 (2020). https://doi.org/10.1007/s00395-020-00836-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00395-020-00836-6