Abstract
Objective
To investigate whether intratumoral heterogeneity, assessed via dynamic contrast-enhanced magnetic resonance imaging (DCE-MRI) and diffusion-weighted imaging (DWI), reflects the molecular subtypes of invasive breast cancers.
Material and methods
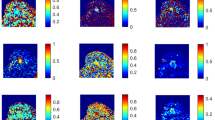
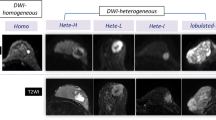
We retrospectively evaluated data from 248 consecutive women (mean age ± standard deviation, 54.6 ± 12.2 years) with invasive breast cancer who underwent preoperative DCE-MRI and DWI between 2019 and 2020. To evaluate intratumoral heterogeneity, kinetic heterogeneity (a measure of heterogeneity in the proportions of tumor pixels with delayed washout, plateau, and persistent components within a tumor) was assessed with DCE-MRI using a commercially available computer-aided diagnosis system. Apparent diffusion coefficients (ADCs) were obtained using a region-of-interest technique, and ADC heterogeneity was calculated using the following formula: (ADCmax−ADCmin)/ADCmean. Possible associations between imaging-based heterogeneity values and breast cancer subtypes were analyzed.
Results
Of the 248 invasive breast cancers, 61 (24.6%) were classified as luminal A, 130 (52.4%) as luminal B, 25 (10.1%) as HER2-enriched, and 32 (12.9%) as triple-negative breast cancer (TNBC). There were significant differences in the kinetic and ADC heterogeneity values among tumor subtypes (p < 0.001 and p = 0.023, respectively). The TNBC showed higher kinetic and ADC heterogeneity values, whereas the HER2-enriched subtype showed higher kinetic heterogeneity values compared to the luminal subtypes. Multivariate linear analysis showed that the HER2-enriched (p < 0.001) and TNBC subtypes (p < 0.001) were significantly associated with higher kinetic heterogeneity values. The TNBC subtype (p = 0.042) was also significantly associated with higher ADC heterogeneity values.
Conclusions
Quantitative assessments of heterogeneity in enhancement kinetics and ADC values may provide biological clues regarding the molecular subtypes of breast cancer.
Key Points
• Higher kinetic heterogeneity was associated with HER2-enriched and triple-negative breast cancer.
• Higher ADC heterogeneity was associated with triple-negative breast cancer.
• Aggressive breast cancer subtypes exhibited higher intratumoral heterogeneity based on MRI.




Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- AUC:
-
Area under the curve
- CAD:
-
Computer-aided diagnosis
- CI:
-
Confidence interval
- DCE:
-
Dynamic contrast-enhanced
- DWI:
-
Diffusion-weighted imaging
- ER:
-
Estrogen receptor
- HER2:
-
Human epidermal growth factor receptor 2
- MRI:
-
Magnetic resonance imaging
- PR:
-
Progesterone receptor
- ROI:
-
Region of interest
- TNBC:
-
Triple-negative breast cancer
References
Bertucci F, Birnbaum D (2008) Reasons for breast cancer heterogeneity. J Biol 7:6
Perou CM, Sørlie T, Eisen MB et al (2000) Molecular portraits of human breast tumours. Nature 406:747–752
Longo DL (2012) Tumor heterogeneity and personalized medicine. N Engl J Med 366:956–957
Nicholson RI, Johnston SR (2005) Endocrine therapy–current benefits and limitations. Breast Cancer Res Treat 93:3–10
Tang P, Skinner KA, Hicks DG (2009) Molecular classification of breast carcinomas by immunohistochemical analysis: are we ready? Diagn Mol Pathol 18:125–132
Trop I, LeBlanc SM, David J et al (2014) Molecular classification of infiltrating breast cancer: toward personalized therapy. Radiographics 34:1178–1195
Höckel M, Knoop C, Schlenger K et al (1993) Intratumoral pO2 predicts survival in advanced cancer of the uterine cervix. Radiother Oncol 26:45–50
Hockel M, Schlenger K, Aral B, Mitze M, Schaffer U, Vaupel P (1996) Association between tumor hypoxia and malignant progression in advanced cancer of the uterine cervix. Cancer Res 56:4509–4515
Waugh S, Purdie C, Jordan L et al (2016) Magnetic resonance imaging texture analysis classification of primary breast cancer. Eur Radiol 26:322–330
Holli-Helenius K, Salminen A, Rinta-Kiikka I et al (2017) MRI texture analysis in differentiating luminal A and luminal B breast cancer molecular subtypes-a feasibility study. BMC Med Imaging 17:69
Kim JY, Kim JJ, Hwangbo L et al (2020) Kinetic heterogeneity of breast cancer determined using computer-aided diagnosis of preoperative MRI scans: relationship to distant metastasis-free survival. Radiology 295:517–526
Kim JY, Kim JJ, Hwangbo L, Kang T, Park H (2019) Diffusion-weighted imaging of invasive breast cancer: relationship to distant metastasis–free survival. Radiology 291:300–307
Fernandez G (2010) Statistical data mining using SAS applications. CRC press
Allred D, Harvey JM, Berardo M, Clark GM (1998) Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod Pathol 11:155–168
Moeder CB, Giltnane JM, Harigopal M et al (2007) Quantitative justification of the change from 10% to 30% for human epidermal growth factor receptor 2 scoring in the American Society of Clinical Oncology/College of American Pathologists guidelines: tumor heterogeneity in breast cancer and its implications for tissue microarray–based assessment of outcome. J Clin Oncol 25:5418–5425
Bustreo S, Osella-Abate S, Cassoni P et al (2016) Optimal Ki67 cut-off for luminal breast cancer prognostic evaluation: a large case series study with a long-term follow-up. Breast Cancer Res Treat 157:363–371
Bae MS, Park SY, Song SE et al (2015) Heterogeneity of triple-negative breast cancer: mammographic, US, and MR imaging features according to androgen receptor expression. Eur Radiol 25:419–427
Navarro Vilar L, Alandete Germán SP, Medina García R, Blanc García E, Camarasa Lillo N, Vilar Samper J (2017) MR imaging findings in molecular subtypes of breast cancer according to BIRADS system. Breast J 23:421–428
Uematsu T, Kasami M, Yuen S (2009) Triple-negative breast cancer: correlation between MR imaging and pathologic findings. Radiology 250:638–647
Youk JH, Son EJ, Chung J, Kim J, Kim E (2012) Triple-negative invasive breast cancer on dynamic contrast-enhanced and diffusion-weighted MR imaging: comparison with other breast cancer subtypes. Eur Radiol 22:1724–1734
Bae MS, Seo M, Kim KG, Park I, Moon WK (2015) Quantitative MRI morphology of invasive breast cancer: correlation with immunohistochemical biomarkers and subtypes. Acta Radiol 56:269–275
Algazzar MAA, Elsayed EE, Alhanafy AM, Mousa WA (2020) Breast cancer imaging features as a predictor of the hormonal receptor status, HER2neu expression and molecular subtype. Egypt J Radiol Nucl Med 51:1–10
Yamaguchi K, Abe H, Newstead GM et al (2015) Intratumoral heterogeneity of the distribution of kinetic parameters in breast cancer: comparison based on the molecular subtypes of invasive breast cancer. Breast Cancer 22:496–502
Leong LCH, Gombos EC, Jagadeesan J, Fook-Chong SMC (2015) MRI kinetics with volumetric analysis in correlation with hormonal receptor subtypes and histologic grade of invasive breast cancers. AJR Am J Roentgenol 204:W348–W356
Blaschke E, Abe H (2015) MRI phenotype of breast cancer: kinetic assessment for molecular subtypes. J Magn Reson Imaging 42:920–924
**e T, Zhao Q, Fu C et al (2019) Differentiation of triple-negative breast cancer from other subtypes through whole-tumor histogram analysis on multiparametric MR imaging. Eur Radiol 29:2535–2544
Montemezzi S, Camera L, Giri MG et al (2018) Is there a correlation between 3T multiparametric MRI and molecular subtypes of breast cancer? Eur J Radiol 108:120–127
Suo S, Cheng F, Cao M et al (2017) Multiparametric diffusion-weighted imaging in breast lesions: association with pathologic diagnosis and prognostic factors. J Magn Reson Imaging 46:740–750
Martincich L, Deantoni V, Bertotto I et al (2012) Correlations between diffusion-weighted imaging and breast cancer biomarkers. Eur Radiol 22:1519–1528
Park SH, Choi H, Hahn SY (2015) Correlations between apparent diffusion coefficient values of invasive ductal carcinoma and pathologic factors on diffusion-weighted MRI at 3.0 tesla. J Magn Reson Imaging 41:175–182
Suo S, Zhang D, Cheng F et al (2019) Added value of mean and entropy of apparent diffusion coefficient values for evaluating histologic phenotypes of invasive ductal breast cancer with MR imaging. Eur Radiol 29:1425–1434
Rakha EA, Martin S, Lee AH et al (2012) The prognostic significance of lymphovascular invasion in invasive breast cancer. Cancer 118:3670–3680
Hennigs A, Riedel F, Gondos A et al (2016) Prognosis of breast cancer molecular subtypes in routine clinical care: a large prospective cohort study. BMC Cancer 16:1–9
Putti TC, Abd El-Rehim DM, Rakha EA et al (2005) Estrogen receptor-negative breast carcinomas: a review of morphology and immunophenotypical analysis. Mod Pathol 18:26–35
Ferrara N (2002) VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer 2:795–803
Mohammed RA, Ellis IO, Mahmmod AM et al (2011) Lymphatic and blood vessels in basal and triple-negative breast cancers: characteristics and prognostic significance. Mod Pathol 24:774–785
Kumar R, Yarmand-Bagheri R (2001) The role of HER2 in angiogenesis. Semin Oncol 28:27–32
Collins DJ, Padhani AR (2004) Dynamic magnetic resonance imaging of tumor perfusion. Approaches and biomedical challenges. IEEE Eng Med Biol Mag 23:65–83
Siemann DW (2011) The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat Rev 37:63–74
Kim EJ, Kim SH, Park GE et al (2015) Histogram analysis of apparent diffusion coefficient at 3.0 t: correlation with prognostic factors and subtypes of invasive ductal carcinoma. J Magn Reson Imaging 42:1666–1678
Seo BK, Pisano ED, Kuzimak CM et al (2006) Correlation of HER-2/neu overexpression with mammography and age distribution in primary breast carcinomas. Acad Radiol 13:1211–1218
Ko ES, Lee BH, Kim H, Noh W, Kim MS, Lee S (2010) Triple-negative breast cancer: correlation between imaging and pathological findings. Eur Radiol 20:1111–1117
Jansen SA, Shimauchi A, Zak L et al (2009) Kinetic curves of malignant lesions are not consistent across MRI systems: need for improved standardization of breast dynamic contrast-enhanced MRI acquisition. AJR Am J Roentgenol 193:832–839
Funding
This study was supported by Biomedical Research Institute Grant (20200268), Pusan National University Hospital.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is ** You Kim.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was waived by the Institutional Review Board.
Ethical approval
Institutional Review Board approval was obtained.
Methodology
• retrospective
• observational
• performed at one institution
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kim, J.J., Kim, J.Y., Suh, H.B. et al. Characterization of breast cancer subtypes based on quantitative assessment of intratumoral heterogeneity using dynamic contrast-enhanced and diffusion-weighted magnetic resonance imaging. Eur Radiol 32, 822–833 (2022). https://doi.org/10.1007/s00330-021-08166-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-021-08166-4