Abstract
Objectives
Develop a CT-based radiomics model and combine it with frozen section (FS) and clinical data to distinguish invasive adenocarcinomas (IA) from preinvasive lesions/minimally invasive adenocarcinomas (PM).
Methods
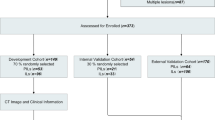
This multicenter study cohort of 623 lung adenocarcinomas was split into training (n = 331), testing (n = 143), and external validation dataset (n = 149). Random forest models were built using selected radiomics features, results from FS, lesion volume, clinical and semantic features, and combinations thereof. The area under the receiver operator characteristic curves (AUC) was used to evaluate model performances. The diagnosis accuracy, calibration, and decision curves of models were tested.
Results
The radiomics-based model shows good predictive performance and diagnostic accuracy for distinguishing IA from PM, with AUCs of 0.89, 0.89, and 0.88, in the training, testing, and validation datasets, respectively, and with corresponding accuracies of 0.82, 0.79, and 0.85. Adding lesion volume and FS significantly increases the performance of the model with AUCs of 0.96, 0.97, and 0.96, and with accuracies of 0.91, 0.94, and 0.93 in the three datasets. There is no significant difference in AUC between the FS model enriched with radiomics and volume against an FS model enriched with volume alone, while the former has higher accuracy. The model combining all available information shows minor non-significant improvements in AUC and accuracy compared with an FS model enriched with radiomics and volume.
Conclusions
Radiomics signatures are potential biomarkers for the risk of IA, especially in combination with FS, and could help guide surgical strategy for pulmonary nodules patients.
Key Points
• A CT-based radiomics model may be a valuable tool for preoperative prediction of invasive adenocarcinoma for patients with pulmonary nodules.
• Radiomics combined with frozen sections could help in guiding surgery strategy for patients with pulmonary nodules.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lung cancer ranks first in cancer mortality around the world [1]. With the popularization of computed tomography (CT) and the application of low-dose CT for lung cancer screening, substantial early-stage lung cancers have been detected [2]. Most malignant pulmonary nodules are confirmed as adenocarcinoma by pathology [3]. Patients with different types of adenocarcinoma differ in 5-year survival probabilities; e.g., patients with a diagnosis of invasive adenocarcinoma (IA) have a significantly poorer survival probability than those with adenocarcinoma in situ (AIS) or minimally invasive adenocarcinoma (MIA), who have a nearly 100% survival probability [4, 5]. Currently, lobectomy may be a better choice than sublobar resection for patients with IA, and patients with preinvasive lesions (atypical adenomatous hyperplasia (AAH) and AIS) and MIA (collectively PM) are candidates for limited resections [6].
Three methods are most commonly used to perform intraoperative or preoperative diagnosis in clinical practice, namely chest CT scan, biopsy, and intraoperative frozen section (FS). Many radiological studies rely on morphological (semantic) features such as spiculation or lobulation to generate a differential diagnosis. However, qualitative interpretation of the image is hampered by the strong subjectivity introduced by atypical radiology signs, especially in small and in ground-glass nodules [7,8,9,10]. Moreover, transbronchial and percutaneous biopsies are limited by the difficulties of sampling and localization [26]. Moreover, a population-based prospective study indicated that the risk factor for develo** lung cancer increases with age and with a family history of lung cancer for female patients [27]. However, in this study, only age and gender significantly differ between cohorts diagnosed with IA and PM, with males older than 60 years having a significantly higher probability to be diagnosed with IA. Age has been reported elsewhere to increase the risk factor of IA diagnosis, while gender differences in the adenocarcinoma spectrum need further study [8,9,10]. Our results also show that a model informed purely on clinical variables has low sensitivity and relatively high specificity for the identification of IA, which may lead to moderate accuracy for diagnosis and low benefit from decision curve. This result, however, should be interpreted with caution, because clinical variables are varied in different populations.
Another study also looked at semantic features, proposing that pulmonary nodules with a larger diameter, located in the upper lobe, spiculation, and PSN (part-solid nodule) had a higher probability to be malignant [27]. However, it has been shown that semi-automated volume analysis is a more robust method than a simple measurement of the diameter to measure the size of the pulmonary nodule [28], and spiculation is an uncommon feature in early-stage lung cancer [8]. Our study finds that nodule diameter and nodule type are significantly different between cohorts diagnosed with IA and PM, with nodules with smaller diameter and pure GGN types increasing the probability of PM diagnosis. These two semantic features by themselves, as well as the semantic model, show high AUC and accuracy values for prediction and diagnosis of IA. Overall, our results indicate both a semantic feature model and a lesion volume model show similar predictive performance compared with radiomics, while radiomics has higher accuracy than semantic and volume models.
It is important also to point out that the ground truth used for diagnosis in this study is fairly unique as resections are not generally considered for pGGNs in guidelines in most countries outside of Asia where pGGNs are followed up until a solid component appears or the tumor progresses [29]. Moreover, pGGN adenocarcinomas are more common in low-risk Asian females than other populations, and the patients more often request surgery. Around 34% of nodules in this study are pGGNs, 30% of which are confirmed as IA, which may reflect doctors’ and patients’ more positive attitudes towards surgery.
In our study, the CT-based radiomics model shows a similar predictive performance with FS in distinguishing IA from PM. Selected features (Wavelet_HLL_Stats_max, Wavelet_LLL_Stats_cov, and LocInt_peakLocal) reflect the distribution of intensity values within the ROI, and another selected feature (GLRLM_LGRE) describes the heterogeneity of the density within the ROI [23]. Lim et al found that the mean density differs between IA and non- or minimally IA [8]. Moreover, a previous study reported that IA tends to appear more heterogeneous on CT images than PM [30]. Therefore, we hypothesize that radiomics features describing density and heterogeneity are related to tumor biology and pathology and are an excellent predictor for identification of IA [25].
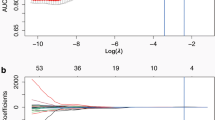
CT and positron emission tomography radiomics studies have shown predictive features could be a surrogate of lesion volume and knowledge of which features correlate highly with volume is therefore important [31,32,33]. Upon volume correlation analysis, we excluded one feature that correlated highly with volume and found no change in model performance. The volume was embedded into the radiomics signature since radiomics is synonymous with quantitative imaging; features that contribute to model performance should not be excluded a priori. In this study, a radiomics plus volume model (RV) showed slight improvement of accuracy compared with the radiomics-alone model, and it had similar AUC and accuracy values as the CSRV model. In addition, we found that our models employing radiomics (i.e., radiomics alone, RV, and CSRV) had similar predictive performance (AUC) as the frozen section models. However, the accuracy of these models was lower than that of FS.
Although the FS can be a precise diagnostic method to guide intraoperative resection procedures for lung adenocarcinoma, it remains difficult to recommend a definitive assessment by FS alone [34]. Borczuk suggested that combining clinical and radiologic information with FS could reduce diagnostic errors [35]. Our results show no significant difference in the AUC values between the FSRV and FSV models, but the former model has better accuracy and calibration. Furthermore, we found that the AUC of the CSFSRV model is not significantly different from that of the FSRV model, did not increase the accuracy, and got bad calibration. In addition, the decision curve indicates that the models containing FS all had better performance than the models without FS. Therefore, we conclude that the addition of radiomics (with volume) to FS analysis potentially creates a substantial biomarker for assessing the risk of invasive adenocarcinomas and could be applied in clinical practice.
Nevertheless, this study has certain limitations. First, because of the retrospective data collection, selection bias is unavoidable. Further prospectively international investigation as a registered clinical trial is paramount. Second, different population cohorts, tumor morphology, and CT parameters are known to influence the results of radiomics features [36]. Further external validation datasets are desired to verify the reliability of our model, especially including diverse cohorts to fully capture phenotype heterogeneity. Third, the ROIs were contoured manually, which is time-consuming and highly prone to error. Therefore, a reliable and robust automatic segmentation tool is necessary to address this issue [37], also taking into account, e.g., peritumoral and normal tissue, to increase the accuracy of quantitative image-based models. Fourth, the accuracy and specificity of the FS analysis in our cohort were lower than the results from previous studies [6, 11]. We speculate that we included more small size and GGN cases, which have lower accuracy than larger tumors as most studies found [6, 11, 12]. Future prospects include prospective validation and deep learning methods for automatic segmentation and in combination with the ones described in this study, novel parametric imaging techniques. While this work focuses on the correlation of radiomics features with the underlying biology (histology), future work will also focus on the prediction of clinical outcomes directly, such as overall survival, progression free survival, or response to therapy.
In conclusion, a radiomics signature can be employed as a preoperative tool to distinguish invasive adenocarcinoma from preinvasive lesions or MIA. Furthermore, a multifactorial model combining radiomics with FS analysis is a potential biomarker for assessing the risk of invasive adenocarcinoma during surgery, and this model could help the therapeutic strategy for patients with pulmonary nodules.
Abbreviations
- AAH:
-
Atypical adenomatous hyperplasia
- AIS:
-
Adenocarcinoma in situ
- AUC:
-
Area under the curve
- CI:
-
Confidence interval
- CT:
-
Computed tomography
- FS:
-
Frozen section
- GGN:
-
Ground-glass nodule
- IA:
-
Invasive adenocarcinoma
- ICC:
-
Intra-/inter-class correlation coefficients
- MIA:
-
Minimally invasive adenocarcinoma
- NPV:
-
Negative predictive values
- pGGN:
-
Pure ground-glass nodule
- PM:
-
Preinvasive lesions or minimally invasive adenocarcinomas
- PPV:
-
Positive predictive values
- PSN:
-
Part-solid nodule
- ROC:
-
Receiver operating characteristic
- ROI:
-
Regions of interest
- ROV:
-
Radiomics plus volume model
- TRIPOD:
-
Transparent Reporting of a Multivariable Prediction Model for Individual Prognosis or Diagnosis
References
Siegel RL, Miller KD, Jemal A (2019) Cancer Statistics, 2019. CA Cancer J Clin 69:7–34
National Lung Screening Trial Research Team, Church TR, Black WC et al (2013) Results of initial low-dose computed tomographic screening for lung cancer. N Engl J Med 368:1980–1991
Maldonado F, Boland JM, Raghunath S et al (2013) Noninvasive characterization of the histopathologic features of pulmonary nodules of the lung adenocarcinoma spectrum using computer-aided nodule assessment and risk yield (CANARY)--a pilot study. J Thorac Oncol 8:452–460
Travis WD, Brambilla E, Noguchi M et al (2011) International association for the study of lung cancer/american thoracic society/european respiratory society international multidisciplinary classification of lung adenocarcinoma. J Thorac Oncol 6:244–285
Yoshizawa A, Motoi N, Riely GJ et al (2011) Impact of proposed IASLC/ATS/ERS classification of lung adenocarcinoma: prognostic subgroups and implications for further revision of staging based on analysis of 514 stage I cases. Mod Pathol 24:653–664
Liu S, Wang R, Zhang Y et al (2016) Precise diagnosis of intraoperative frozen section is an effective method to guide resection strategy for peripheral small-sized lung adenocarcinoma. J Clin Oncol 34:307–313
Nakamura H, Saji H, Shinmyo T et al (2015) Close association of IASLC/ATS/ERS lung adenocarcinoma subtypes with glucose-uptake inpositron emission tomography. Lung Cancer 87:28–33
Lim HJ, Ahn S, Lee KS et al (2013) Persistent pure ground-glass opacity lung nodules /=10 mm in diameter at CT scan: histopathologic comparisons and prognostic implications. Chest 144:1291–1299
Lee HJ, Goo JM, Lee CH et al (2009) Predictive CT findings of malignancy in ground-glass nodules on thin-section chest CT: the effects on radiologist performance. Eur Radiol 19:552–560
Lee KH, Goo JM, Park SJ et al (2014) Correlation between the size of the solid component on thin-section CT and the invasive component on pathology in small lung adenocarcinomas manifesting as ground-glass nodules. J Thorac Oncol 9:74–82
Zhu E, **e H, Dai C et al (2018) Intraoperatively measured tumor size and frozen section results should be considered jointly to predict the final pathology for lung adenocarcinoma. Mod Pathol 31:1391–1399
Yeh YC, Nitadori J, Kadota K et al (2015) Using frozen section to identify histological patterns in stage I lung adenocarcinoma of ≤3 cm: accuracy and interobserver agreement. Histopathology 66:922–938
Group of Respiration Diseases, Chinese Society of Pathology (2019) Consensus on early stage non-mucinous lepidic lung adenocarcinoma frozen section diagnosis. Zhonghua Bing Li Xue Za Zhi 48:3–10
Lambin P, Rios-Velazquez E, Leijenaar R et al (2012) Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer 48:441–446
Aerts HJ, Velazquez ER, Leijenaar RT et al (2014) Decoding tumour phenotype by noninvasive imaging using a quantitative radiomics approach. Nat Commun 5:4006
Fan L, Fang M, Li Z et al (2019) Radiomics signature: a biomarker for the preoperative discrimination of lung invasive adenocarcinoma manifesting as a ground-glass nodule. Eur Radiol 29:889–897
She Y, Zhang L, Zhu H et al (2018) The predictive value of CT-based radiomics in differentiating indolent from invasive lung adenocarcinoma in patients with pulmonary nodules. Eur Radiol 28:5121–5128
Chae HD, Park CM, Park SJ, Lee SM, Kim KG, Goo JM (2014) Computerized texture analysis of persistent part-solid ground-glass nodules: differentiation of preinvasive lesions from invasive pulmonary adenocarcinomas. Radiology 273:285–293
Lee SM, Park CM, Goo JM, Lee HJ, Wi JY, Kang CH (2013) Invasive pulmonary adenocarcinomas versus preinvasive lesions appearing as ground-glass nodules: differentiation by using CT features. Radiology 268:265–273
Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J (2008) Fleischner Society: glossary of terms for thoracic imaging. Radiology 246:697–722
Bankier AA, MacMahon H, Goo JM, Rubin GD, Schaefer-Prokop CM, Naidich DP (2017) Recommendations for measuring pulmonary nodules at CT: a statement from the Fleischner Society. Radiology 285:584–600
Shafiq-Ul-Hassan M, Zhang GG, Latifi K et al (2017) Intrinsic dependencies of CT radiomic features on voxel size and number of gray levels. Med Phys 44:1050–1062
Lambin P, Leijenaar RTH, Deist TM et al (2017) Radiomics: the bridge between medical imaging and personalized medicine. Nat Rev Clin Oncol 14:749–762
Moons KG, Altman DG, Reitsma JB et al (2015) Transparent Reporting of a multivariable prediction model for Individual Prognosis or Diagnosis (TRIPOD): explanation and elaboration. Ann Intern Med 162:W1–W73
Sanduleanu S, Woodruff HC, de Jong EEC et al (2018) Tracking tumor biology with radiomics: a systematic review utilizing a radiomics quality score. Radiother Oncol 127:349–360
Powell HA, Iyen-Omofoman B, Hubbard RB, Baldwin DR, Tata LJ (2013) The association between smoking quantity and lung cancer in men and women. Chest 143:123–129
McWilliams A, Tammemagi MC, Mayo JR et al (2013) Probability of cancer in pulmonary nodules detected on first screening CT. N Engl J Med 369:910–919
Heuvelmans MA, Walter JE, Vliegenthart R et al (2018) Disagreement of diameter and volume measurements for pulmonary nodule size estimation in CT lung cancer screening. Thorax 73:779–781
Bai C, Choi CM, Chu CM et al (2016) Evaluation of pulmonary nodules: Clinical Practice Consensus Guidelines for Asia. Chest 150:877–893
Ost DE, Gould MK (2012) Decision making in patients with pulmonary nodules. Am J Respir Crit Care Med 185:363–372
Welch ML, McIntosh C, Haibe-Kains B et al (2019) Vulnerabilities of radiomic signature development: the need for safeguards. Radiother Oncol 130:2–9
Orlhac F, Soussan M, Maisonobe JA, Garcia CA, Vanderlinden B, Buvat I (2014) Tumor texture analysis in 18F-FDG PET: relationships between texture parameters, histogram indices, standardized uptake values, metabolic volumes, and total lesion glycolysis. J Nucl Med 55:414–422
Ibrahim A, Vallières M, Woodruff H et al (2019) Radiomics analysis for clinical decision support in nuclear medicine. Semin Nucl Med 49:438–449
Chen D, Dai C, Kadeer X, Mao R, Chen Y, Chen C (2018) New horizons in surgical treatment of ground-glass nodules of the lung: experience and controversies. Ther Clin Risk Manag 14:203–211
Borczuk AC (2017) Challenges of frozen section in thoracic pathology: lepidic lesions, limited resections, and margins. Arch Pathol Lab Med 141:932–939
Berenguer R, Pastor-Juan MDR, Canales-Vázquez J et al (2018) Radiomics of CT features may be nonreproducible and redundant: influence of CT acquisition parameters. Radiology 288:407–415
Charbonnier JP, Chung K, Scholten ET et al (2018) Automatic segmentation of the solid core and enclosed vessels in subsolid pulmonary nodules. Sci Rep 8:646
Funding
This study was financially supported by the program of China Scholarships Council (n° 201808210318), ERC advanced grant (ERC-ADG-2015, n° 694812 - Hypoximmuno), and ERC-2018-PoC (n° 81320 – CL-IO). This research is also supported by the Dutch technology Foundation STW (grant n° P14-19 Radiomics STRaTegy), which is the applied science division of NWO, and the Technology Programme of the Ministry of Economic Affairs. This is also financially supported by the SME Phase 2 (RAIL - n°673780), EUROSTARS (DART, DECIDE, COMPACT), the European Program H2020-2015-17 (ImmunoSABR - n° 733008, PREDICT - ITN - n° 766276), TRANSCAN Joint Transnational Call 2016 (JTC2016 “CLEARLY”- n° UM 2017-8295), Interreg V-A Euregio Meuse-Rhine (“Euradiomics”), and Kankeronderzoekfonds Limburg (KOFL) from the Health Foundation Limburg and the Dutch Cancer Society.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Guarantor
The scientific guarantor of this publication is Philippe Lambin.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Statistics and biometry
Two of the authors have significant statistical expertise.
Informed consent
The requirement for informed consent was waived and patient data are anonymized.
Ethical approval
The institutional review boards approved this retrospective study registered in http://clinicaltrials.gov (identifier: NCT03872362).
Methodology
• Retrospective
• Diagnostic or prognostic study
• Multicenter study
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 454 kb)
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Wu, G., Woodruff, H.C., Sanduleanu, S. et al. Preoperative CT-based radiomics combined with intraoperative frozen section is predictive of invasive adenocarcinoma in pulmonary nodules: a multicenter study. Eur Radiol 30, 2680–2691 (2020). https://doi.org/10.1007/s00330-019-06597-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-019-06597-8