Abstract
Objective
To determine the diagnostic characteristics of poor visualisation of nigrosome-1 as a neuroimaging biomarker in Parkinson’s disease (PD) and to explore the relationship of poor visualisation of nigrosome-1 and clinical asymmetry.
Methods
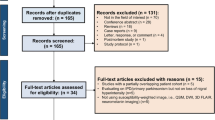
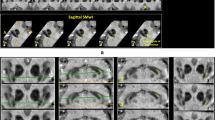
High-resolution gradient-echo sequences of 67 patients with PD and 63 healthy controls were reviewed by two radiologists blinded to the clinical details. A three-tier classification system was used to categorise the scans based on the visualisation of nigrosome-1, and inter-rater reliability was calculated at each level of classification. Other diagnostic properties such as sensitivity, specificity and predictive values were calculated. The relationship between poor visualisation of nigrosome-1 and clinical asymmetry was also assessed.
Results
Poor visualisation of nigrosome-1 had high sensitivity (98.5%), specificity (93.6%), positive-predictive value (94.3%), negative-predictive value (98.3%), accuracy (96%) and inter-rater reliability (k = 0.75–0.92). Poorly visualised nigrosome-1 was significantly associated with higher motor asymmetry in the contralateral side in 64.8% of subjects (p = 0.004).
Conclusions
Poor visualisation of nigrosome-1 in PD had good diagnostic properties as a neuroimaging biomarker in PD. There was also a significant agreement on clinical asymmetry and poor visualisation of nigrosome-1.
Key Points
• Nigrosome-1 represents the largest collection of dopaminergic neurons in dorso-lateral substantia nigra.
• Loss of nigrosome-1 is being studied as a biomarker in Parkinson’s disease.
• Visualisation of nigrosome-1 had good diagnostic properties as a biomarker.
• There was a contralateral relationship between nigrosome-1 lateralisation and clinical asymmetry.
• We also highlight the potential limitations of nigrosome-1 visualisation as a biomarker.



Similar content being viewed by others
Abbreviations
- 3D:
-
3-Dimensional space
- Group 1:
-
Well visualised on both sides
- Group 2:
-
Poorly visualised on one or both sides
- Group 2a:
-
Poorly visualised unilaterally
- Group 2a-L:
-
Poorly visualised on left side
- Group 2a-R:
-
Poorly visualised on right side
- Group 2b:
-
Poorly visualised bilaterally
- k:
-
Cohen’s kappa
- MRI:
-
Magnetic resonance imaging
- MSA-P:
-
Multisystem atrophy – Parkinsonian type
- PD:
-
Parkinson’s disease
- PSP:
-
Progressive supranuclear palsy
- SENSE:
-
Sensitivity Encoding for Fast MRI
- SPECT:
-
Single Photon Emission Computed Tomography
- UPDRS III:
-
Unified Parkinson’s Disease Rating Scale part III
- VenoBOLD sequence:
-
Venous blood oxygen level-dependent sequence
References
Hughes AJ, Daniel SE, Kilford L, Lees AJ (1992) Accuracy of clinical diagnosis of idiopathic Parkinson’s disease. A clinico-pathological study of 100 cases. JNNP 55:181–184
Hughes AJ, Daniel SE, Lees AJ (2001) Improved accuracy of clinical diagnosis of Lewy body Parkinson’s disease. Neurology 57:1497–1499
Schrag A, Ben-Shlomo Y, Quinn N (2002) How valid is the clinical diagnosis of Parkinson’s disease in the community? J Neurol Neurosurg Psychiatry 73:529–534
Meara J, Bhowmick BK, Hobson P (1999) Accuracy of diagnosis in patients with presumed Parkinson’s disease. Age Ageing 28:99–102
Hauser RA, Grosset DG (2011) [(123) I] FP-CIT (DaTscan) SPECT Brain imaging in patients with suspected parkinsonian syndromes. J Neuroimaging Off J Am Soc Neuroimaging. 1–6. doi:10.1111/j.1552-6569.2011.00583.x
Bajaj N, Hauser RA, Grachev ID (2013) Clinical utility of dopamine transporter single photon emission CT (DaT-SPECT) with (123I) ioflupane in diagnosis of parkinsonian syndromes. J Neurol Neurosurg Psychiatry 84:1288–1295
Caudle WM, Bammler TK, Lin Y, Pan S, Zhang J (2010) Using “omics” to define pathogenesis and biomarkers of Parkinson’s disease. Expert Rev Neurother 10:925–942
Miller DB, O’Callaghan JP (2014) Biomarkers of Parkinson’s Disease (PD): present and future. Metabolism 64:S40–S46
Wang J, Hoekstra JG, Zuo C, Cook TJ, Zhang J (2013) Biomarkers of Parkinson’s disease: current status and future perspectives. Drug Discov Today 18:155–162
Ogisu K, Kudo K, Sasaki M, Sakushima K, Yabe I, Sasaki H et al (2013) 3D neuromelanin-sensitive magnetic resonance imaging with semi- automated volume measurement of the substantia nigra pars compacta for diagnosis of Parkinson's disease. Neuroradiology 55:719–724
Castellanos G, Fernández-Seara MA, Lorenzo-Betancor O, Ortega-Cubero S, Puigvert M, Uranga J et al (2015) Automated neuromelanin imaging as a diagnostic biomarker for Parkinson's disease. Mov Disord 30:945–952
Damier P, Hirsch EC, Agid Y, Graybiel AM (1999) The substantia nigra of the human brain: II. Patterns of loss of dopamine-containing neurons in Parkinson’s disease. Brain 122:1437–1448
Schwarz ST, Afzal M, Morgan PS, Bajaj N, Gowland PA, Auer DP (2014) The “swallow tail” appearance of the healthy nigrosome - A new accurate test of Parkinson’s disease: a case-control and retrospective cross-sectional MRI study at 3T. PLoS One. 9 doi:10.1371/journal.pone.0093814.
Cosottini M, Frosini D, Pesaresi I et al (2015) Comparison of 3T and 7T susceptibility-weighted angiography of the substantia nigra in diagnosing Parkinson disease. Am J Neuroradiol 36:461–466
Blazejewska AI, Schwarz ST, Pitiot A et al (2013) Visualization of nigrosome 1 and its loss in PD: pathoanatomical correlation and in vivo 7 T MRI. Neurology 81:534–540
Noh Y, Sung YH, Lee J, Kim EY (2015) nigrosome 1 detection at 3T MRI for the diagnosis of early-stage idiopathic Parkinson disease: assessment of diagnostic accuracy and agreement on imaging asymmetry and clinical laterality. Am J Neuroradiol 36:2010–2016
Kim J, Jeong H, Jung Y et al (2016) Loss of substantia nigra hyperintensity on 7 Tesla MRI of Parkinson’s disease, multiple system atrophy, and progressive supranuclear palsy, Park Relat Disord. 1–8. doi:10.1016/j.parkreldis.2016.01.023.
Mueller C, Pinter B, Reiter E et al (2014) Dorsolateral hyperintense nigral sign on SWI at 3T MRI. Mov Disord 29:S86
Reiter E, Mueller C,. Pinter B et al (2015) Dorsolateral nigral hyperintensity on 3.0T susceptibility-weighted imaging in neurodegenerative Parkinsonism. Mov Disord doi:10.1002/mds.26171
Jha M, Jhunjhunwala K, Sankara BB, Saini J, Kumar JK, Yadav R et al (2015) Neuropsychological and imaging profile of patients with Parkinson's disease and freezing of gait. Parkinsonism Relat Disord 21:1184–1190
Naduthota RM, Bharath RD, Jhunjhunwala K, Yadav R, Saini J, Christopher R et al (2017) Imaging biomarker correlates with oxidative stress in Parkinson's disease. Neurol India 65:263–268
Uitti RJ, Baba Y, Whaley NR, Wszolek ZK, Putzke JD (2005) Parkinson disease: handedness predicts asymmetry. Neurology 64:1925–1930
Barrett MJ, Wylie SA, Harrison MB, Wooten GF (2011) Handedness and motor symptom asymmetry in Parkinson’s disease. J Neurol Neurosurg Psychiatry 82:1122–1124
Daniel Maxim L, Niebo R, Utell MJ (2014) Screening tests: a review with examples. Inhal Toxicol 26:811–828
Lehéricy S, Bardinet E, Poupon C, Vidailhet M, François C (2014) 7 tesla magnetic resonance imaging: a closer look at substantia nigra anatomy in Parkinson’s disease. Mov Disord 29:1574–1581
Verclytte S, Fisch O, Colas L, Vanaerde O, Toledano M, Budzik JF (2017) ASL and susceptibility-weighted imaging contribution to the management of acute ischaemic stroke. Insights Imaging 8:91–100
Bae YJ, Kim JM, Kim E et al (2016) Loss of nigral hyperintensity on 3 Tesla MRI of Parkinsonism: comparison with 123I-FP-CIT SPECT. Mov Disord. doi:10.1002/mds.26584
Sung YH, Noh Y, Lee J, Kim EY (2015) Drug-induced Parkinsonism versus idiopathic parkinson disease: utility of nigrosome 1 with 3-T imaging. Radiology. doi:10.1148/radiol.2015151466
Kamagata K, Nakatsuka T, Sakakibara R, Tsuyusaki Y, Takamura T, Sato K et al (2017) Diagnostic imaging of dementia with Lewy bodies by susceptibility-weighted imaging of nigrosomes versus striatal dopamine transporter single-photon emission computed tomography: a retrospective observational study. Neuroradiology 59:89–98
De Marzi R, Seppi K, Högl B, Müller C, Scherfler C, Stefani A et al (2016) Loss of dorsolateral nigral hyperintensity on 3.0 tesla susceptibility-weighted imaging in idiopathic rapid eye movement sleep behavior disorder. Ann Neurol 79:1026–1030
Gerlach M, Double KL, Ben-Shachar D, Zecca L, Youdim MBH, Riederer P (2003) Neuromelanin and its interaction with iron as a potential risk factor for dopaminergic neurodegeneration underlying Parkinson’s disease. Neurotox Res 5:35–43
Zecca L, Casella L, Albertini A et al (2008) Neuromelanin can protect against iron-mediated oxidative damage in system modeling iron overload of brain aging and Parkinson’s disease. J Neurochem 106:1866–1875
Oh SW, Shin NY, Lee JJ, Lee SK, Lee PH, Lim SM et al (2016) Correlation of 3D FLAIR and dopamine transporter inaging in patient with Parkinsonism. Am J Roentgenol 207:1089–1094
Meijer FJ, Steens SC, van Rumund A, van Cappellen van Walsum AM, Küsters B, Esselink RA et al (2016) nigrosome-1 on susceptibility weighted imaging to differentiate Parkinson's disease from atypical parkinsonism: an in vivo and ex vivo pilot study. Pol J Radiol 3:81–363 –9
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Guarantor
The scientific guarantor of this publication is Dr. Pramod Kumar Pal.
Conflict of interest
The authors of this manuscript declare no relationships with any companies whose products or services may be related to the subject matter of the article.
Funding
This work was financially supported by Department of Biotechnology (DBT), Government of India. [Grant Number: NO. BT/PR14315/MED/30/474/2010]
Statistics and biometry
No complex statistical methods were necessary for this paper.
Informed consent
Written informed consent was obtained from all subjects in this study.
Ethical approval
Institutional Review Board approval was obtained.
Study subjects or cohorts overlap
There is overlap of subjects from the following previously published reports/articles:
1. Jha M, Jhunjhunwala K, Sankara BB, Saini J, Kumar JK, Yadav R, Pal PK (2015) Neuropsychological and imaging profile of patients with Parkinson's disease and freezing of gait. Parkinsonism Relat Disord 21(10):1184-90. doi: 10.1016/j.parkreldis.2015.08.009. Epub 2015 Aug 12.
2. Naduthota RM, Bharath RD, Jhunjhunwala K, Yadav R, Saini J, Christopher R, Pal PK (2017) Imaging biomarker correlates with oxidative stress in Parkinson's disease. Neurol India(2017). 65(2):263-268. doi: 10.4103/neuroindia.NI_981_15.
Methodology
• Prospective
• Case-control study
• Performed at one institution
Rights and permissions
About this article
Cite this article
Stezin, A., Naduthota, R.M., Botta, R. et al. Clinical utility of visualisation of nigrosome-1 in patients with Parkinson’s disease. Eur Radiol 28, 718–726 (2018). https://doi.org/10.1007/s00330-017-4950-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00330-017-4950-5