Abstract
Consequences of Corona Virus Disease-19 (COVID-19) in patients with rheumatic diseases (RDs) are clinically diverse. SARS-CoV-2 infection has been associated with various autoimmune and rheumatic manifestations over the past three years. Emerging evidence points to the possibility of Long COVID predisposition in rheumatic patients due to the changes in immune regulatory response. The aim of this article was to overview data on the pathobiology of Long COVID in patients with RDs. Related risk factors, clinical characteristics, and prognosis of Long COVID in RDs were analyzed. Relevant articles were retrieved from Medline/PubMed, Scopus, and Directory of Open Access Journals (DOAJ). Diverse mechanisms of viral persistence, chronic low-grade inflammation, lasting production of autoantibodies, endotheliopathy, vascular complications, and permanent tissue damage have been described in association with Long COVID. Patients with RDs who survive COVID-19 often experience severe complications due to the immune disbalance resulting in multiple organ damage. Regular monitoring and treatment are warranted in view of the accumulating evidence.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Three years into the Corona Virus Disease-19 (COVID-19) pandemic, caused by Severe Acute Respiratory Syndrome Corona Virus 2 (SARS-CoV-2), has resulted in unprecedented global issues with more than 0.6 billion cases of the disease and 6.7 billion deaths (as of February 1, 2023) [1]. Numerous countries have faced several waves of COVID-19 outbreaks with enormous financial and social implications.
Despite the improved understanding of the acute phase of the disease, its long-term consequences are still poorly explored. One-third of COVID-19 survivors are repeatedly hospitalized over a mean period of 140-day follow-up [2]. And up to one-third of survivors experience a variety of symptoms after the acute phase of COVID-19 [3, 4], raising concerns of uncontrolled complications.
The underlying pathobiology of COVID-19 long-term consequences remains poorly explored while an increasing number of patients with rheumatic diseases (RDs) and COVID-19 complications are encountered in the rheumatologist's practice. The association between SARS-CoV-2 and RDs is seemingly bidirectional. There is a risk of COVID‐19 advancement in subjects with RDs [5]. On the other hand, COVID-19 itself may trigger autoimmunity and result in new rheumatic manifestations [6].
There are scarce data on outcomes of COVID-19 in patients with RDs. Better understanding of prolonged COVID-19 symptoms in patients with RDs may help improve monitoring and treatment. With that in mind, we aimed to analyze pathogenic mechanisms of COVID-19 symptoms and phenotypes in patients with RDs over a long term.
Search strategy
We searched through Medline/PubMed, Scopus, and Directory of Open Access Journals (DOAJ) databases for relevant original articles, case studies, and reviews published by February 1, 2023. The following keywords were employed: post-COVID-19, post-acute COVID-19 syndrome, Long COVID, rheumatic diseases, immune-mediated inflammatory disease. Our search strategy was in line with previously published recommendations [7].
COVID-19 features in patients with RDs
SARS-CoV-2 is an enveloped single-stranded RNA virus with a weight of approximately 29.9 kB and a diameter of 50–200 nm [8]. The virus penetrates human cells by binding to angiotensin-converting enzyme-related carboxypeptidase (ACE2) receptor which is expressed on numerous organs, including the lungs, intestines, brain, heart, kidneys, and liver [9]. It enters its target cells after being cleaved by the transmembrane protease serine 2 (TMPRSS2) via viral spike proteins 1 and 2 [10]. ACE2 is downregulated after binding with virus, significantly contributing to cytokine-mediated hyperinflammatory response [11].
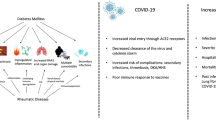
Clinical characteristics of COVID-19 range from asymptomatic or mild course to multiorgan failure (MOF) and death. A Chinese study of 44.000 COVID-19-positive subjects demonstrated that 81% of patients had mild symptoms, 14% developed severe symptoms, and 5% progressed to life-threatening conditions [12]. Timely diagnosis of COVID-19 is critically important in subjects with underlying immune disbalance and high activity of RDs [3, 13]. A US-based retrospective study revealed that patients with RDs have a threefold higher incidence of coronavirus infection than the general population. Moreover, SARS-CoV-2 positivity significantly increases the risk of RD flares (Odds Ratio [OR] 4.6, 95% Confidence Interval [CI] 1.2–17.4) [14]. Likewise, patients with immune-mediated inflammatory diseases (IMIDs) and COVID-19 presented with an increased hospitalization risk compared to non-IMID controls (adjusted OR [aOR] 1.2) [15]. Patients with iritis (OR 1.5), psoriatic arthritis (PsA) (OR 2), rheumatoid arthritis (RA) (OR 1.4), and vasculitis (OR 2.1) were also at risk of hospitalization due to COVID-19 [16]. Severe COVID-19 has been associated with RDs. In fact, an increased frequency of respiratory failure was reported in patients with RDs and COVID-19 as compared to non-RD controls (38% vs 10%, p < 0.001) [17].
Various RD phenotypes may confound clinical outcomes of COVID-19. Notably, the Global Rheumatology Alliance reported a greater risk of worse COVID-19 outcomes in patients with giant-cell arteritis at older age (OR 1.9, 95% CI 1.3–2.8) and those with obesity (OR 3, 95% CI 1.2–7.6) [15]. Similarly, older age (OR 1.6, 95% CI 1.3–1.9), rituximab (RTX) use (OR 2.15, 95% CI 1.15–4.01), cyclophosphamide use (OR 4.3, 95% CI 1.1–16.8) were associated with severe COVID-19 in subjects with antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis (AAV) [15]. Therefore, there is a need for RD phenotype stratification to adjust treatment strategies and avoid worse COVID-19 outcomes [15].
Hyperinflammatory response in COVID-19
Immune dysregulation merits special attention in subjects with COVID-19 [18]. Available evidence points to an interaction of SARS-CoV-2 with innate immunity via pattern recognition receptors (PRRs) such as Toll-Like Receptors (TLRs) or Retinoic acid-inducible gene I (RIG-I)-like from the components receptors (RLRs) [19]. The interaction is followed by downstream signaling with secretion of various cytokines, including type I/III interferons (IFNs), tumor necrosis factor-alpha (TNF-alpha), interleukin-1 (IL-1), and IL-6, resulting in adaptive immune responses [20]. An augmented inflammatory response with an increase in IL-6/IL-10 ratio may lead to adverse outcomes [21]. However, regulated production of IFN-I counteracts SARS-CoV-2 infection [20]. Overall, an uncontrolled increase in cytokines during the acute period of COVID-19 manifests as cytokine release syndrome (CRS) and MOF and heightens the risk of autoimmune complications at a later stage [22].
Immune mechanisms in Long COVID
More than 20% of subjects surviving acute COVID-19 may suffer from some persisting symptoms of infection and develop new ones after one month [23]. This clinical condition is known as post-COVID-19 syndrome (PCS) or Long COVID that frequently presents with fatigue, musculoskeletal signs, cognitive impairment, and sleep disorders [23]. Although here is no universally accepted definition of Long COVID-19, it can be characterized as a systemic inflammatory response develo** during or after SARS-CoV-2 infection, lasting from 3 to 12 months, and not attributable to any alternative diagnosis [24].
Long COVID manifests as a clinically variable condition. A sizeable proportion of Long COVID patients develop rheumatic symptoms [25]. Older age (OR 1.2, 95% CI 1.1–1.4, per 10-year increase) and female gender (OR 1.6, 95% CI 1.1–2.2) increase the risk of rheumatic symptoms [25]. Emerging evidence considers immune dysregulation as the key pathogenic feature of Long COVID. A case series of Long COVID-19 revealed significantly elevated TNF-α, IL-1β, and IL-13 and significantly decreased interferon-γ-induced protein 10 (IP-10) as compared to the onset of COVID-19 [26]. In view of immunological shifts, IgG anti-SARS-CoV-2 S1 antibodies remained significantly elevated from the acute phase of COVID-19 and persisted in Long COVID [26]. Lymphocytes, including Th9, CD8 + effector T cells, B cells, and CD4 + effector memory cells also remained increased compared to pre-pandemic controls and to levels measured at day 28 of COVID-19 [26]. Upregulation of proinflammatory mediators appeared to be maintained in COVID survivors even 8 months after the infection [27]. Persistent overexpression of type I IFN (IFN-β) and type III IFN (IFN-λ1) and high proinflammatory cytokines such as IFN-β, IFN-γ, and IL-6 linked to Long COVID with 78.5–81.6% accuracy [27]. This chronic inflammatory response with elevated B cells, known as the primary source of autoantibodies accounts for the likelihood of autoimmune mechanisms in long-term COVID complications [26].
Autoimmunity in COVID-19 and Long COVID
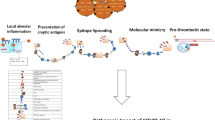
Some of the reported autoimmune manifestations of Long COVID can be induced by viral persistence, endothelial dysfunction, and altered levels of inflammatory biomarkers after the acute phase of COVID-19 [28]. Numerous autoantibodies, including antinuclear antibodies (ANA) and ANCA, have been found in patients infected with SARS-CoV-2 [29,30,31]. Chang et al. reported that approximately 50% of subjects hospitalized with SARS-CoV-2 positivity had autoantibodies linked with various RDs such as myositis and systemic sclerosis [32]. Another study reported the frequency of anti-52 kDa SSA/Ro, anti-60 kDa SSA/Ro, and ANA antibodies (20%, 25%, and 50%, respectively) in patients with COVID-19, suggesting the activation of the autoimmunity [33].
One of the proposed mechanisms of Long COVID-19 involves the formation of neutrophil extracellular traps (NETs) that may create the environment for autoantibody production with the exposure of intranuclear components of cells [34]. Another mechanism of Long COVID associates autoimmunity with pathobiology of B cells. In severe COVID-19 extrafollicular B-cell activation strongly correlated with poor disease prognosis and elevated SARS-CoV-2 specific neutralizing antibodies [35]. A case of post-COVID-19 systemic lupus erythematosus (SLE) with accompanying B-cell activation was reported [36]. Another study revealed that 83% of patients with Long COVID develop latent autoimmunity and 62% present with polyautoimmunity. More than 85% of patients had anti-SARS-CoV-2 IgG antibodies that positively correlated with autoantibodies, age, and body mass index (BMI) [37]. Prevention of Long COVID triggering mechanisms remains poorly understood (Fig. 1).
Long COVID symptoms over time
Long COVID clinical features are variable over time (Table 1). A meta-analysis by Lopez-Leon et al. reported tiredness, headache, hair loss, and dyspnea as the most frequently seen symptoms in Long COVID. However, this report did not distinguish sets of symptoms in hospitalized and non-hospitalized subjects and did not relate to follow-up periods [38]. Based on the COVID-19 Global Rheumatology Alliance Vaccine Survey of subjects with systemic autoimmune rheumatic diseases (SARDs), 1 in 4 had COVID-19 symptoms lasting 28 days or longer and 1 in 10 experienced the same 90 days or longer. The following factors were associated with prolonged symptom complexes: hospitalization for COVID-19 (age-aOR 6.5, 95% CI 3.0–14.1), comorbidities (aOR 1.1 per comorbidity, 95% CI 1.0–1.2), and osteoarthritis (aOR 2.1, 95% CI 1.0–4.3) [39].
Two-month follow-up of patients recovering from SARS-CoV-2 infection
Two months after the recovery from COVID-19 pneumonia, almost half of survivors demonstrated restrictive lung disease while a reduced lung diffusion capacity (DLCO) was reported in one-third of cases [40].
Three-month follow-up of patients recovering from SARS-CoV-2 infection
Three months after the acute phase, 64% (n = 35) of patients still suffered from COVID-19-related symptoms and 71% (n = 39) showed various degrees of radiological and physiological pulmonary abnormalities [64].
Endotheliopathy and Long COVID
Persisting endotheliopathy is a typical feature of Long COVID [65]. SARS-CoV-2 damages the endothelium by targeting ACE2 receptor [66]. The resultant endotheliopathy underlies multisystemic features in COVID-19 [67].
Markers of endothelial cell (EC) activation, including von Willebrand factor antigen (VWF: Ag), VWF pro-peptide (VWFpp), and factor VIII significantly increase in convalescent COVID-19 subjects compared to healthy controls [65]. Thrombogenic factors associated with increased COVID-19-related mortality include elevated D-dimer at hospital admission with an OR of 18.4 (95% CI 2.6–128.5) [68]. However, Moreno-Perez et al. reported that CRP and D-dimer were not predictive of Long COVID [69].
Pulmonary impairment and Long COVID
A meta-analysis of 16 cohort studies with hospitalized patients followed more than 1 month post-discharge or more than 2 months post-admission reported 20% of pulmonary disturbances. Diffusion impairment was the most frequent feature, followed by restrictive ventilatory defects [70]. Acute respiratory distress syndrome (ARDS) associated with COVID-19 may contribute to the lasting damage of alveoli with irreversible fibrosis [71]. Cytokine storm as the trigger of immunopathological pathways may be associated with pulmonary fibrosis in Long COVID [72].
COVID-19 may contribute to thromboembolic microangiopathy with subsequent immunomodulatory reactions in the pulmonary vascular bed. These mechanisms may result in chest pain in post-COVID-19 periods [73]. Overall, individuals who recovered from COVID-19 with residual pulmonary injury should be monitored for at least 36 months [74].
Potential biomarkers of Long COVID
Increasing cases of Long COVID draw the public health system's attention to seek diagnostic markers identifying illness progression and timely follow-up after hospital discharge. SARS-CoV-2 spike protein might justify its diagnostic value. S1 subunit of SARS-CoV-2 spike protein is detected in about 65% of patients with Long COVID during a one-year follow-up period [75]. Dysregulation of the neuropilin-1 (NRP-1)/vascular endothelial growth factor (VEGF)-A pathway may also underly the course of Long COVID. In a cohort study, about 50% (n = 48) of individuals with COVID complications had significantly increased serum levels of VEGF compared to fully recovered subjects [76]. More studies are warranted to correctly screen, diagnose, and manage Long COVID.
Treatment of Long COVID
RTX therapy has been associated with the risk of severe COVID-19 in patients with RDs; subjects on RTX therapy have frequently presented with critical conditions (effect size 3.3, 95% CI 1.7–6.4) and experienced a longer hospital stay (0.6, 95% CI 0.5–0.9) in comparison with subjects not treated with RTX [77]. Long-term RTX therapy reduces levels of B-cells with subsequent low antibody production. This effect may cause the impairment of viral clearance by antibodies [78].
HCQ was another widely used DMARD for COVID-19 at the beginning of the pandemic. Initial reports were optimistic over the use of HCQ to treat SARS-CoV-2 infection [79] suggesting that low doses of HCQ were relatively safe for patients with RDs infected with SARS-CoV-2 [80]. A clinical trial investigating HCQ with and without azithromycin versus placebo reported reduced viral load/disappearance in patients with severe disease [81]. However, side effects of high doses of HCQ were described in COVID-19 patients [82]. Cardiotoxicity with arrhythmias reported in the elderly with pre-existing cardiac history questioned the safety and efficacy of HCQ therapy [83]. Another trial demonstrated that hospitalized COVID-19 subjects on HCQ did not show a lower incidence of death at 28 days compared to non-HCQ subjects, confirming the lack of HCQ efficacy in COVID-19 [84]. The role of HCQ in the context of COVID-19 long-term complications has not been examined. The potential utility of HCQ in Long COVID could be due to its inhibition of TLR signaling, binding to their ligands, and dampening proinflammatory cytokine production [85].
GCT has attracted more attention in the COVID-19 pandemic. Patients with RDs on ≥ 10 mg per day systemic GCT presented with increased odds of positive SARS-CoV-2 test (aOR 1.47, 95% CI 1.05–2.03), severe COVID-19 (OR 1.76, 95% CI 1.06–2.96), and COVID-19-related death (OR 3.34, 95% CI 1.23–8.9) [86]. Careful dose adjustment may prevent poor clinical outcomes in COVID-19 due to the immune-modulating effect [86]. One study identified the effects of long-term GCT on the incidence and outcomes of COVID-19 in patients with RDs [13]. A univariate analysis revealed that moderate doses of glucocorticoids (GCs) (7.5–20 mg) conferred a higher risk of COVID-19 (RR = 1.7, 95% CI 1.1- 2.6) that persisted in a multivariate analysis (RR 1.6, 95% CI 1.0- 2.5) [13].
A large case series of the COVID-19 Global Rheumatology Alliance reported an association between GC dose ≥ 10 mg/daily and hospitalization (OR 2.05, 95% CI 1.06–3.96) [5]. However, there was no association with hospitalization for DMARD alone or in combination with biologics/Janus Kinase inhibitors [5]. Likewise, there was no association for non-steroidal anti-inflammatory drugs (NSAID) [5].
Notably, corticosteroid therapies in the pandemic may increase the frequency of avascular necrosis (AVN), particularly with high doses and longer duration of therapies. A recent case series showed the presence of AVN in patients with COVID-19 treated with prednisolone at mean dose of 758 mg (400 mg–1250 mg) [87] that was less than the mean cumulative dose reported in the literature [88]. Careful monitoring of patients with hip and thigh pain and timely bisphosphonate therapy after acute COVID-19 may help avoid severity complications through [87]. Finally, GC use may increase the risk of thrombosis in patients exposed to SARS-CoV-2 [89].
Discontinuation of treatment during the COVID-19 pandemic
Many patients with RDs discontinued their therapies due to the fear of SARS-CoV-2 infection [90, 91]. There was a significant difference in disease activity in those who discontinued their treatment and those who continued (p = 0.001) [28].
In a case report of a 37-year-old female with history of RA, antiviral therapy with nirmatrelvir/ritonavir reduced Long COVID symptoms; meanwhile, discontinuation of tocilizumab therapy triggered new-onset brain damage [92]. Rational and informed discussions about the risks of withdrawal effects may help to optimize the recovery in Long COVID.
Vaccination and COVID-19
An Italian multicentric cohort study has shown that vaccinated patients were less likely to suffer from Long COVID [93]. Patient education was shown to increase awareness of vaccine safety and efficiency and to reduce related fears [94].
A UK-based study analyzed the association between COVID-19 vaccination and post-COVID-19 symptoms in patients previously infected with SARS-CoV-2 [95]. The first vaccine dose reduced the risk of post-COVID-19 symptoms by 13% within a median follow-up of 141 days. The second dose decreased the same risk by 9% within a median follow-up of 67 days [95]. Overall, COVID-19 vaccination decreases the risk of Long COVID.
Common side effects of COVID-19 vaccination include pain at the injection site, redness, paresthesia, and fatigue; more serious adverse effects are vaccine-induced immune thrombotic thrombocytopenia (VITT) and cerebral venous sinus thrombosis (CVST) [96]. The mechanism of VITT could be due to the interaction between free DNA in the vaccines and platelet factor 4 (PF4), leading to PF4-reactive autoantibodies production [96]. In addition, post-COVID-19 vaccination was associated with a sixfold greater risk of gout flare within three months (aOR 6.02, 95% CI 3.00–12.08); colchicine prophylaxis showed its effectiveness due to lowering the risk of gout flare by 47% after vaccination [97]. Another research reported the possible association between the development of lupus anticoagulant-associated venous thromboembolism and Pfizer mRNA COVID-19 vaccination [98].One study reported 5 cases of RDs after the second dose of Pfizer-BioNTech and Moderna COVID-19 vaccinations [99]. Further studies are required to elucidate the pathogenesis and possible connection between COVID-19 mRNA vaccination and the likelihood of RDs occurrence.
Conclusions
Considering a high frequency of Long COVID, more efforts are needed to raise public awareness of mitigating COVID-19 lasting consequences in RDs. Numerous questions remain unanswered regarding the prevention of immune complications over a prolonged period in patients with RDs.
Dampening prolonged inflammatory reactions and persistent autoantibody production may help avoid COVID-19 lasting complications. Rheumatologists should closely monitor COVID-19 survivors with lasting symptoms and personalize diagnostic and therapeutic approaches.
Data Availability
Data supporting the findings of this study are available from the corresponding author [Fedorchenko Yu.V.] on request.
References
COVID Live—coronavirus statistics—Worldometer. https://www.worldometers.info/coronavirus/#countries. Accessed 1st February 2023
Ayoubkhani D, Khunti K, Nafilyan V, Maddox T, Humberstone B, Diamond I et al (2021) Post-covid syndrome in individuals admitted to hospital with covid-19: retrospective cohort study. BMJ 372:n693. https://doi.org/10.1136/bmj.n693
Thronicke A, Hinse M, Weinert S, Jakubowski A, Grieb G, Matthes H (2022) Factors associated with self-reported post/Long-COVID-a real-world data study. Int J Environ Res Public Health 19(23):16124. https://doi.org/10.3390/ijerph192316124
Ledford H (2022) How common is Long COVID? Why studies give different answers. Nature 606(7916):852e3. https://doi.org/10.1038/d41586-022-01702-2
Gianfrancesco M, Hyrich KL, Al-Adely S et al (2020) Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis 79:859–866. https://doi.org/10.1136/annrheumdis-2020-217871
Dotan A, Muller S, Kanduc D, David P, Halpert G, Shoenfeld Y (2021) The SARS-CoV-2 as an instrumental trigger of autoimmunity. Autoimmun Rev 20(4):102792. https://doi.org/10.1016/j.autrev.2021.102792
Gasparyan AY, Ayvazyan L, Blackmore H, Kitas GD (2011) Writing a narrative biomedical review: considerations for authors, peer reviewers, and editors. Rheumatol Int 31:1409. https://doi.org/10.1007/s00296-011-1999-324
Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y et al (2020) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395:507–513. https://doi.org/10.1016/s0140-6736(20)30211-7
South AM, Diz DI, Chappell MC (2020) COVID-19, ACE2, and the cardiovascular consequences. Am J Physiol Heart Circ Physiol 318:H1084–H1090. https://doi.org/10.1152/ajpheart.00217.2020
Jackson CB, Farzan M, Chen B, Choe H (2022) Mechanisms of SARS-CoV-2 entry into cells. Nat Rev Mol Cell Biol 23(1):3–20. https://doi.org/10.1038/s41580-021-00418-x
Perico L, Benigni A, Casiraghi F, Ng LFP, Renia L, Remuzzi G (2021) Immunity, endothelial injury and complement-induced coagulopathy in COVID-19. Nat Rev Nephrol 17(1):46–64
Wu Z, McGoogan JM (2020) Characteristics of and important lessons from the coronavirus disease 2019 (COVID-19) outbreak in China: summary of a report of 72314 cases from the Chinese Center For Disease Control And Prevention. JAMA 323(13):1239–1242
Patil A, Chanakya K, Shenoy P, Chandrashekara S, Haridas V, Kumar S et al (2022) A prospective longitudinal study evaluating the influence of immunosuppressives and other factors on COVID-19 in autoimmune rheumatic diseases. BMC Rheumatol 6(1):32. https://doi.org/10.1186/s41927-022-00264-0
Fike A, Hartman J, Redmond C, Williams SG, Ruiz-Perdomo Y, Chu J et al (2021) Risk factors for COVID-19 and rheumatic disease flare in a US cohort of latino patients. Arthritis Rheumatol 73(7):1129–1134. https://doi.org/10.1002/art.41656
Sattui SE, Conway R, Putman MS, Seet AM, Gianfrancesco MA, Beins K et al (2021) Outcomes of COVID-19 in patients with primary systemic vasculitis or polymyalgia rheumatica from the COVID-19 Global rheumatology alliance physician registry: a retrospective cohort study. Lancet Rheumatol 3(12):e855–e864. https://doi.org/10.1016/S2665-9913(21)00316-7
Eder L, Croxford R, Drucker AM, Mendel A, Kuriya B, Touma Z et al (2022) COVID-19 hospitalizations, intensive care unit stays, ventilation, and death among patients with immune-mediated inflammatory diseases compared to controls. J Rheumatol 49(5):523–530. https://doi.org/10.3899/jrheum.211012
Ye C, Cai S, Shen G, Guan H, Zhou L, Hu Y et al (2020) Clinical features of rheumatic patients infected with COVID-19 in Wuhan. China Ann Rheum Dis 79(8):1007–1013. https://doi.org/10.1136/annrheumdis-2020-217627
Novelli L, Motta F, De Santis M, Ansari AA, Gershwin ME, Selmi C (2021) The JANUS of chronic inflammatory and autoimmune diseases onset during COVID-19—a systematic review of the literature. J Autoimmun 117:102592
Yamada T, Takaoka A (2023) Innate immune recognition against SARS-CoV-2. Inflamm Regen 43(1):7. https://doi.org/10.1186/s41232-023-00259-5
Kindler E, Thiel V (2016) SARS-CoV and IFN: too little, too late. Cell Host Microbe 19:139–141. https://doi.org/10.1016/j.chom.2016.01.012
McElvaney OJ, Hobbs BD, Qiao D, McElvaney OF, Moll M, McEvoy NL et al (2020) A linear prognostic score based on the ratio of interleukin-6 to interleukin-10 predicts outcomes in COVID-19. EBioMedicine 61:103026. https://doi.org/10.1016/j.ebiom.2020.103026
Wang J, Jiang M, Chen X, Montaner LJ (2020) Cytokine storm and leukocyte changes in mild versus severe SARS-CoV-2 infection: review of 3939 COVID-19 patients in China and emerging pathogenesis and therapy concepts. J Leukoc Biol 108(1):17–41
Anaya J-M, Rojas M, Salinas ML et al (2021) Post-COVID syndrome. A case series and comprehensive review. Autoimmun Rev 20:102947
Baimukhamedov C (2022) How long is Long COVID. Int J Rheum Dis. https://doi.org/10.1111/1756-185X.14494
Cui D, Wang Y, Huang L, Gu X, Huang Z, Mu S et al (2022) Rheumatic symptoms following coronavirus disease 2019 (COVID-19): a chronic post-COVID-19 condition. Open Forum Infect Dis 9(6):ofac170. https://doi.org/10.1093/ofid/ofac170
Acosta-Ampudia Y, Monsalve DM, Rojas M, Rodríguez Y, Zapata E, Ramírez-Santana C et al (2022) Persistent autoimmune activation and proinflammatory state in post-coronavirus disease 2019 syndrome. J Infect Dis 225(12):2155–2162. https://doi.org/10.1093/infdis/jiac017
Phetsouphanh C, Darley DR, Wilson DB, Howe A, Munier CML, Patel SK et al (2022) Immunological dysfunction persists for 8 months following initial mild-to-moderate SARS-CoV-2 infection. Nat Immunol 23(2):210–216. https://doi.org/10.1038/s41590-021-01113-x
Rivera J, Rodríguez T, Pallarés M, Castrejón I, González T, Vallejo-Slocker L et al (2022) Prevalence of post-COVID-19 in patients with fibromyalgia: a comparative study with other inflammatory and autoimmune rheumatic diseases. BMC Musculoskelet Disord 23(1):471. https://doi.org/10.1186/s12891-022-05436-0
Gazzaruso C, Carlo Stella N, Mariani G et al (2020) High prevalence of antinuclear antibodies and lupus anticoagulant in patients hospitalized for SARS-CoV2 pneumonia. Clin Rheumatol 39(7):2095–2097
Sacchi MC, Tamiazzo S, Stobbione P, Agatea L, De Gaspari P, Stecca A et al (2021) SARS-CoV-2 infection as a trigger of autoimmune response. Clin Transl Sci 14(3):898–907. https://doi.org/10.1111/cts.12953
Gracia-Ramos AE, Martin-Nares E, Hernandez-Molina G (2021) New onset of autoimmune diseases following COVID-19 diagnosis. Cells 10(12):3592
Chang SE, Feng A, Meng W, Apostolidis SA, Mack E, Artandi M et al (2021) New-onset IgG autoantibodies in hospitalized patients with COVID-19. medRxiv. https://doi.org/10.1101/2021.01.27.21250559
Zhou Y, Han T, Chen J, Hou C, Hua L, He S (2020) Clinical and autoimmune characteristics of severe and clinical cases of COVID-19. Clin Transl Sci 13:1077–1086
Essien F, Chastant L, McNulty C, Hubbard M, Lynette L, Carroll M (2022) COVID-19-induced psoriatic arthritis: a case report. Ther Adv Chronic Dis 13:20406223221099332. https://doi.org/10.1177/20406223221099333
Woodruff M, Ramonell R, Nguyen D et al (2020) Extrafollicular B cell responses correlate with neutralizing antibodies and morbidity in COVID-19. Nat Immunol 21:1506–1516
Ramachandran L, Dontaraju VS, Troyer J, Sahota J (2022) New onset systemic lupus erythematosus after COVID-19 infection: a case report. AME Case Rep 6:14. https://doi.org/10.21037/acr-21-55
Deeks JJ, Dinnes J, Takwoingi Y, Davenport C, Spijker R, Taylor-Phillips S et al (2020) Antibody tests for identification of current and past infection with SARS-CoV-2. Cochrane Database Syst Rev 6(6):CD013652. https://doi.org/10.1002/14651858.CD013652
Lopez-Leon S, Wegman-Ostrosky T, Perelman C, Sepulveda R, Rebolledo PA, Cuapio A, et al (2021) More than 50 long-term effects of COVID-19: a systematic review and meta-analysis. medRxiv. 2021. 2021.01.27.21250617.
DiIorio M, Kennedy K, Liew JW, Putman MS, Sirotich E, Sattui SE et al (2022) Prolonged COVID-19 symptom duration in people with systemic autoimmune rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance Vaccine Survey. RMD Open 8(2):e002587. https://doi.org/10.1136/rmdopen-2022-002587
Strumiliene E, Zeleckiene I, Bliudzius R, Samuilis A, Zvirblis T, Zablockiene B et al (2021) Follow-up analysis of pulmonary function, exercise capacity, radiological changes, and quality of life two months after recovery from SARS-CoV-2 pneumonia. Medicina 57(6):568. https://doi.org/10.3390/medicina57060568
Zhao YM, Shang YM, Song WB, Li QQ, **e H, Xu QF et al (2020) Follow-up study of the pulmonary function and related physiological characteristics of COVID-19 survivors three months after recovery. EClinicalMedicine 25:100463. https://doi.org/10.1016/j.eclinm.2020.100463
van den Borst B et al (2020) Comprehensive health assessment three months after recovery from acute COVID-19. Clin Infect Dis 73(5):e1089–e1098
Huang C, Huang L, Wang Y, Li X, Ren L, Gu X et al (2021) 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet 397(10270):220–232. https://doi.org/10.1016/S0140-6736(20)32656-8
Davis HE et al (2021) Characterizing Long COVID in an international cohort: 7 months of symptoms and their impact. EClinicalMedicine 38:101019
Zhang S, Bai W, Yue J, Qin L, Zhang C, Xu S et al (2021) Eight months follow-up study on pulmonary function, lung radiographic, and related physiological characteristics in COVID-19 survivors. Sci Rep 11(1):13854. https://doi.org/10.1038/s41598-021-93191-y
Kingery JR, Safford MM, Martin P, Lau JD, Rajan M, Wehmeyer GT et al (2022) Health status, persistent symptoms, and effort intolerance one year after acute COVID-19 infection. J Gen Intern Med 37(5):1218–1225. https://doi.org/10.1007/s11606-021-07379-z
Zhao Y, Yang C, An X, **ong Y, Shang Y, He J et al (2021) Follow-up study on COVID-19 survivors one year after discharge from hospital. Int J Infect Dis 112:173–182. https://doi.org/10.1016/j.ijid.2021.09.017
Conway R, Nikiphorou E, Demetriou CA, Low C, Leamy K, Ryan JG et al (2023) Outcomes of COVID-19 in people with rheumatic and musculoskeletal disease in Ireland over the first 2 years of the pandemic. Ir J Med Sci 9:1–6. https://doi.org/10.1007/s11845-022-03265-7
Fernández-de-Las-Peñas C, Rodríguez-Jiménez J, Fuensalida-Novo S, Palacios-Ceña M, Gómez-Mayordomo V, Florencio LL et al (2021) Myalgia as a symptom at hospital admission by severe acute respiratory syndrome coronavirus 2 infection is associated with persistent musculoskeletal pain as long-term post-COVID sequelae: a case-control study. Pain 162(12):2832–2840. https://doi.org/10.1097/j.pain.0000000000002306
Taha SI, Samaan SF, Ibrahim RA, El-Sehsah EM, Youssef MK (2021) Post-COVID-19 arthritis: is it hyperinflammation or autoimmunity? Eur Cytokine Netw 32(4):83–88. https://doi.org/10.1684/ecn.2021.0471
Zubchenko S, Kril I, Nadizhko O, Matsyura O, Chopyak V (2022) Herpesvirus infections and post-COVID-19 manifestations: a pilot observational study. Rheumatol Int 42(9):1523–1530. https://doi.org/10.1007/s00296-022-05146-9
Shukla AK, Atal S, Banerjee A, Jhaj R, Balakrishnan S, Chugh PK et al (2023) An observational multi-centric COVID-19 sequelae study among health care workers. Lancet Reg Health Southeast Asia. 10:100129. https://doi.org/10.1016/j.lansea.2022.100129
Hooper JE, Uner M, Priemer DS, Rosenberg A, Chen L (2021) Muscle biopsy findings in a case of sars-cov-2-associated muscle injury. J Neuropathol Exp Neurol 80(4):377–378. https://doi.org/10.1093/jnen/nlaa155
Lechien JR, Chiesa-Estomba CM, De Siati DR, Horoi M, Le Bon SD, Rodriguez A et al (2020) Olfactory and gustatory dysfunctions as a clinical presentation of mild-to-moderate forms of the coronavirus disease (COVID-19): a multicenter European study. Eur Arch Otorhinolaryngol 277:2251–2261. https://doi.org/10.1007/s00405-020-05965-1
Montes-Ibarra M, Oliveira CLP, Orsso CE, Landi F, Marzetti E, Prado CM (2022) The impact of Long COVID-19 on muscle health. Clin Geriatr Med 38(3):545–557. https://doi.org/10.1016/j.cger.2022.03.004
Ursini F, Ciaffi J, Mancarella L, Lisi L, Brusi V, Cavallari C et al (2021) Fibromyalgia: a new facet of the post-COVID-19 syndrome spectrum? Results from a web-based survey. RMD Open 7(3):e001735. https://doi.org/10.1136/rmdopen-2021-001735
Tiantian C, **g Z, Yiwen M, Yiming Y, Haifeng Y, Feng H et al (2023) COVID-19 causing death in a rheumatoid arthritis patient who retested positive for SARS-CoV-2 RNA: a case report. Int J Rheum Dis. https://doi.org/10.1111/1756-185X.14555
Quinones-Moya H, Valle AO, Camargo-Coronel A, Jimenenez-Balderas FJ, Bernal-Enriquez MB, Madinabeitia-Rodríguez P et al (2023) Long COVID in patients with rheumatologic disease: a single center observational study. Indian J Rheumatol. https://doi.org/10.4103/injr.injr_118_22
Mormile I, Mormile M, Rea G, Petraroli A, Barbieri V, de Paulis A, Rossi FW (2022) Spontaneous pneumo-mediastinum in a post-COVID-19 patient with systemic sclerosis. Healthcare 10(3):529. https://doi.org/10.3390/healthcare10030529
Yalcin Mutlu M, Taubmann J, Wacker J, Tascilar K, Fagni F, Gerner M et al (2022) Neutralizing monoclonal antibodies against SARS-CoV-2 for COVID-19 pneumonia in a rituximab treated patient with systemic sclerosis-A case report and literature review. Front Med 9:934169. https://doi.org/10.3389/fmed.2022.934169
Fineschi S (2021) Case report: systemic sclerosis after covid-19 infection. Front Immunol 12:686699. https://doi.org/10.3389/fimmu.2021.686699
Chandra A, Kahaleh B (2022) Systemic sclerosis (SSc) after COVID-19: a case report. Cureus. 14(3):e23179. https://doi.org/10.7759/cureus.23179
Nocturne G, Mariette X (2013) Advances in understanding the pathogenesis of primary Sjögren’s syndrome. Nat Rev Rheumatol 9:544–556. https://doi.org/10.1038/nrrheum.2013.110
Brito-Zerón P, Acar-Denizli N, Romão VC, Armagan B, Seror R, Carubbi F et al (2021) Post-COVID-19 syndrome in patients with primary Sjögren’s syndrome after acute SARS-CoV-2 infection. Clin Exp Rheumatol 39(Suppl 133 6):57–65. https://doi.org/10.55563/clinexprheumatol/0anatx
Fogarty H, Townsend L, Morrin H, Ahmad A, Comerford C, Karampini E et al (2021) Persistent endotheliopathy in the pathogenesis of Long COVID syndrome. J Thromb Haemost 19(10):2546–2553. https://doi.org/10.1111/jth.15490
Hoffmann M, Kleine-Weber H, Schroeder S, Krüger N, Herrler T, Erichsen S et al (2020) SARS-CoV-2 cell entry depends on ACE2 and TMPRSS2 and is blocked by a clinically proven protease inhibitor. Cell 181(2):271-280.e8. https://doi.org/10.1016/j.cell.2020.02.052
Hamming I, Timens W, Bulthuis ML, Lely AT, Navis G, van Goor H (2004) Tissue distribution of ACE2 protein, the functional receptor for SARS coronavirus. A first step in understanding SARS pathogenesis. J Pathol 203(2):631–637. https://doi.org/10.1002/path.1570
Zhou F, Yu T, Du R et al (2020) Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 395(10229):1054–1062
Moreno-Pérez O, Merino E, Leon-Ramirez JM et al (2021) Post-acute COVID-19 syndrome Incidence and risk factors: a mediterranean cohort study. J Infect 82(3):378–383. https://doi.org/10.1016/j.**f.2021.01.004
Long Q, Li J, Hu X, Bai Y, Zheng Y, Gao Z (2021) Follow-ups on persistent symptoms and pulmonary function among post-acute COVID-19 patients: a systematic review and meta-analysis. Front Med 8:702635. https://doi.org/10.3389/fmed.2021.702635
British Society for Immunology (2020) Long-Term Immunological Health Consequences of COVID-19. Available at https://www.immunology.org/sites/default/files/BSI_Briefing_Note_August_2020_FINAL.pdf.
Ali RMM, Ghonimy MBI (2021) Post-COVID-19 pneumonia lung fibrosis: a worrisome sequelae in surviving patients. Egypt J Radiol Nucl Med 52:101
Dhawan RT, Gopalan D, Howard L, Vicente A, Park M, Manalan K et al (2021) Beyond the clot: perfusion imaging of the pulmonary vasculature after COVID-19. Lancet Respir Med 9:107–116
Raghu G (2020) Wilson KC (2020) COVID-19 interstitial pneumonia: monitoring the clinical course in survivors. Lancet Respir Med 8(9):839–842. https://doi.org/10.1016/S2213-2600(20)30349-0
Swank Z, Senussi Y, Manickas-Hill Z, Yu XG, Li JZ, Alter G et al (2022) Persistent circulating severe acute respiratory syndrome coronavirus 2 spike is associated with post-acute coronavirus disease 2019 sequelae. Clin Infect Dis ciac. https://doi.org/10.1093/cid/ciac722
Torres-Ruiz J, Lomelín-Gascón J, Lira-Luna J, Pérez-Fragoso A, Tapia-Conyer R, Nuñez-Aguirre M et al (2021) FANSY POSTCOV: a composite clinical immunological predictive index for post-covid-19 syndrome unveils distinctive features in a cohort study of mild to critical patients. Clin Transl Med 11:e623. https://doi.org/10.1002/ctm2.623
Avouac J, Drumez E, Hachulla E, Seror R, Georgin-Lavialle S, El Mahou S et al (2021) COVID-19 outcomes in patients with inflammatory rheumatic and musculoskeletal diseases treated with rituximab: a cohort study. Lancet Rheumatol 3(6):e419–e426. https://doi.org/10.1016/S2665-9913(21)00059-X
Wölfel R, Corman VM, Guggemos W et al (2020) Virological assessment of hospitalized patients with COVID-2019. Nature 581:465–469
Tang W, Cao Z, Han M, Wang Z, Chen J, Sun W et al (2020) Hydroxychloroquine in patients with mainly mild to moderate coronavirus disease 2019: open label, randomised controlled trial. BMJ 369:m1849. https://doi.org/10.1136/bmj.m1849
Majewski D, Majewska KA, Naskręcka M, Grygiel-Górniak B (2021) Chloroquine and hydroxychloroquine—safety profile of potential COVID-19 drugs from the rheumatologist’s perspective. Ann Agric Environ Med 28(1):122–126. https://doi.org/10.26444/aaem/127766
Gautret P, Lagier J-C, Parola P et al (2020) Hydroxychloroquine and azithromycin as a treatment of COVID-19: results of an open-label non-randomized clinical trial. Int J Antimicrob Agents. https://doi.org/10.1016/j.ijantimicag.2020.105949
Fihn SD, Perencevich E, Bradley SM (2020) Caution needed on the use of chloroquine and hydroxychloroquine for coronavirus disease 2019. JAMA Netw Open 3(4.23):e209035. https://doi.org/10.1001/jamanetworkopen.2020.9035
Dos Reis Neto ET, Kakehasi AM, de Medeiros PM, Ferreira GA, Marques CDL, da Mota LMH et al (2020) Revisiting hydroxychloroquine and chloroquine for patients with chronic immunity-mediated inflammatory rheumatic diseases. Adv Rheumatol 60(1):32. https://doi.org/10.1186/s42358-020-00134-8
RECOVERY Collaborative Group, Horby P, Mafham M, Linsell L, Bell JL, Staplin N et al (2020) Effect of hydroxychloroquine in hospitalized patients with Covid-19. N Engl J Med 383(21):2030–2040. https://doi.org/10.1056/NEJMoa2022926
Wang SSY, Xu C (2023) Hydroxychloroquine: is there a role in long COVID? Clin Rheumatol 25:1–2. https://doi.org/10.1007/s10067-023-06514-x
Shin YH, Shin JI, Moon SY, ** HY, Kim SY, Yang JM et al (2021) Autoimmune inflammatory rheumatic diseases and COVID-19 outcomes in South Korea: a nationwide cohort study. Lancet Rheumatol 3(10):e698–e706. https://doi.org/10.1016/S2665-9913(21)00151-X
Agarwala SR, Vijayvargiya M, Pandey P (2021) Avascular necrosis as a part of “Long COVID-19.” BMJ Case Rep 14(7):e242101. https://doi.org/10.1136/bcr-2021-242101
McKee MD, Waddell JP, Kudo PA, Schemitsch EH, Richards RR (2001) Osteonecrosis of the femoral head in men following short-course corticosteroid therapy: a report of 15 cases. CMAJ 164(2):205–206
Monti M, Vertogen B, Masini C, Donati C, Lilli C, Zingaretti C et al (2020) Hydroxychloroquine as prophylaxis for COVID-19: a review. Front Pharmacol 11:605185. https://doi.org/10.3389/fphar.2020.605185
Coskun BN, Yagiz B, Pehlivan Y, Dalkilic E (2021) Attitudes of patients with a rheumatic disease on drug use in the COVID-19 pandemic. Adv Rheumatol 61(1):55. https://doi.org/10.1186/s42358-021-00211-6
Rosenbaum JT, Hamilton H, Choi D, Weisman MH, Reveille JD, Winthrop KL (2020) Biologics spondylitis and COVID-19. Ann Rheum Dis 79(12):1663–1665. https://doi.org/10.1136/annrheumdis-2020-217941
Visvabharathy L, Orban ZS, Koralnik IJ (2022) Case report: treatment of long COVID with a SARS-CoV-2 antiviral and IL-6 blockade in a patient with rheumatoid arthritis and SARS-CoV-2 antigen persistence. Front Med 9:1003103. https://doi.org/10.3389/fmed.2022.1003103
Azzolini E et al (2022) (2022) Association between BNT162b2 vaccination and Long COVID after infections not requiring hospitalization in health care workers. JAMA 328(7):676–678
Felten R, Dubois M, Ugarte-Gil MF, Chaudier A, Kawka L, Bergier H et al (2021) Vaccination against COVID-19: expectations and concerns of patients with autoimmune and rheumatic diseases. Lancet Rheumatol 3(4):e243–e245. https://doi.org/10.1016/S2665-9913(21)00039-4
Ayoubkhani D, Bermingham C, Pouwels KB, Glickman M, Nafilyan V, Zaccardi F et al (2022) Trajectory of long covid symptoms after covid-19 vaccination: community based cohort study. BMJ 377:e069676. https://doi.org/10.1136/bmj-2021-069676
Sharifian-Dorche M, Bahmanyar M, Sharifian-Dorche A, Mohammadi P, Nomovi M, Mowla A (2021) Vaccine-induced immune thrombotic thrombocytopenia and cerebral venous sinus thrombosis post COVID-19 vaccination; a systematic review. J Neurol Sci 428:117607. https://doi.org/10.1016/j.jns.2021.117607
Lu J, He Y, Terkeltaub R, Sun M, Ran Z, Xu X et al (2022) Colchicine prophylaxis is associated with fewer gout flares after COVID-19 vaccination. Ann Rheum Dis 81(8):1189–1193. https://doi.org/10.1136/annrheumdis-2022-222199
Al-Ahmad M, Al Rasheed M, Altourah L, Rodriguez-Bouza T, Shalaby N (2023) Lupus anticoagulant activity and thrombosis post COVID-19 vaccination. Blood Coagul Fibrinolysis 34(1):75–78. https://doi.org/10.1097/MBC.0000000000001161
VanDerVeer SJ, Maier KD, Hill EM (2022) Rheum-CoV-2 vaccination case series. J Clin Rheumatol. https://doi.org/10.1097/RHU.0000000000001906
Funding
None.
Author information
Authors and Affiliations
Contributions
Conceptualization: FY. Formal analysis: FY, ZO. Writing—original draft: FY. Writing—review and editing: FY, ZO.
Corresponding author
Ethics declarations
Conflict of interest
Both authors have no potential conflicts of interest to disclose. “All of the byline authors meet the ICMJE criteria for authorship. We well understand privilege and responsibility of the authorship of the scientific publications. We declare that we are kee** global and/or local guidelines of research and publication ethics strictly including authorship.”
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Fedorchenko, Y., Zimba, O. Long COVID in autoimmune rheumatic diseases. Rheumatol Int 43, 1197–1207 (2023). https://doi.org/10.1007/s00296-023-05319-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00296-023-05319-0