Abstract
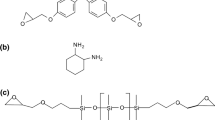
The influence of the end groups of two liquid rubbers on curing kinetics, morphology, and hardness behavior of diglycidyl ether of bisphenol-A based epoxy resin (DGEBA) has been studied. The rubbers are silyl-dihydroxy terminated (PDMS-co-DPS-OH) and silyl-diglycidyl ether terminated (PDMS-DGE). Crosslinking reactions, investigated by shear rheometry, ranged 90–110 °C, using a constant concentration (5 phr) of liquid rubbers and 1,2-Diamino cyclohexane (1,2-DCH) as hardener agent. The gel time, t gel, of the neat epoxy significantly decreased when adding the elastomers, more so for the silyl-dihydroxy terminated elastomer; at 110 °C the reaction was nearly complete before rheological test started. The results suggest that the elastomers induced a catalytic effect on the curing reaction. Scanning electron microscopy revealed phase separation of the elastomer during the curing reaction with rubber domains about 5 μm size. However, the DGEBA/dihydroxy terminated elastomer composite cured at 110 °C exhibited a homogenous morphology, that is, the rapid reaction time would not allow for phase separation. Water contact angle tests evidenced either more hydrophilic (silyl-diglycidyl ether terminated rubber) or more hydrophobic (silyl-dihydroxy terminated rubber) behavior than the neat epoxy. The latter effect is attributed to the presence of aromatic rings in the backbone structure of PDMS-co-DPS-OH. Microindentation measurements show that the elastomers significantly reduced the hardness of the epoxy resin, the DGEBA/ether terminated composite exhibiting the lowest hardness values. Moreover, hardness increased as reaction temperature did, correlating with a reduction of microdomains size thus enabling the tuning of mechanical properties with reaction temperature.














Similar content being viewed by others
References
Winter HH (1987) Can the gel point of a cross-linking polymer be detected by the G′–G′′ crossover? Polym Eng Sci 27:1698–1702
Winter HH (1987) Evolution of rheology during chemical gelation. Progr Col Poly Sci 75:104–110
Larson RG (1999) The structure and rheology of complex fluids, 2nd edn. Oxford University Press, Oxford
Winter HH, Chambon F (1986) Analysis of linear viscoelasticity of a crosslinking polymer at the gel point. J Rheol 30:367–382
Tung C-YM, Dynes PJ (1982) Relationship between viscoelastic properties and gelation in thermosetting systems. J Appl Polym Sci 27:569–574
Núñez L, Gómez-Barreiro S, Gracia-Fernández CA (2005) Study of the influence of isomerism on the curing properties of the epoxy system DGEBA (n = 0)/1,2-DCH by rheology. Rheol Acta 45:184–191
Rudin A (1999) The elements of polymer science and engineering, 2nd edn. Academic Press, New York, p 11
Liu P, He L, Song J, Liang X, Ding H (2008) Microstructure and thermal properties of silyl-terminated polycaprolactone-polysiloxane modified epoxy resin composites. J Appl Polym Sci 109:1105–1113
Laza JM, Julian CA, Larrauri E, Rodríguez M, León LM (1999) Thermal scanning rheometer analysis of curing kinetic of an epoxy resin: 2. An amine as curing agent. Polymer 40:35–45
Carrozzino S, Levita G, Rolla P, Tombari E (1990) Calorimetric and microwave dielectric monitoring of epoxy resin cure. Polymer Eng Sci 30:366–373
Vyazovkin S, Sbirrazzuoli N (1996) Mechanism and kinetics of epoxy-amine cure studied by differential scanning calorimetry. Macromolecules 29:1867–1873
Zhao F, Sun Q, Fang DP, Yao K (2000) Preparation and properties of polydimethylsiloxane-modified epoxy resins. J Appl Polym Sci 76:1683–1690
Hou S-S, Chung Y-P, Chan C-K, Kuo P-L (2000) Function and performance of silicone copolymer. Part IV. Curing behavior and characterization of epoxy-siloxane copolymers blended with diglycidyl ether of bisphenol-A. Polymer 41:3263–3272
Rey L, Poisson N, Maazouz A, Sautereau H (1999) Enhancement of crack propagation resistance in epoxy resins by introducing poly(dimethylsiloxane) particles. J Mater Sci 34:1775–1781
Könczol L, Döll W, Buchholz U, Mülhaupt R (1994) Ultimate properties of epoxy resins modified with a polysiloxane-polycaprolactone block copolymer. J Appl Polym Sci 54:815–826
Anand Prabu A, Alagar M (2005) Thermal and morphological properties of silicone-polyurethane-epoxy intercrosslinked matrix materials. J Macromol Sci Part A Pure Appl Chem 42:175–188
Lin S-T, Huang S-K (1996) Preparation and structural determination of siloxane-modified sulfone-containing epoxy resins. J Polym Sci Part A Polym Chem 34:869–884
Lin S-T, Huang S-K (1996) Synthesis and impact properties of siloxanes-DGEBA epoxy copolymers. J Polym Sci Part A Polym Chem 34:1907–1922
Tripathi G, Srivastava D (2007) Effect of carboxyl-terminated poly(butadiene-co-acrylonitrile) (CTBN) concentration on thermal and mechanical properties of binary blends of diglycidyl ether of bisphenol-A (DGEBA) epoxy resin. Mat Sci Eng A 443:262–269
Ramos VD, da Costa HM, Soares VLP, Nascimento RSV (2005) Modification of epoxy resin: a comparison of different types of elastomers. Polym Test 24:387–394
Tripathi G, Srivastava D (2009) Toughened cycloaliphatic epoxy resin for demanding thermal applications and surface coatings. J Appl Polym Sci 114:2769–2776
Thomas R, Durix S, Sinturel C, Omonov T, Goossens S, Groeninckx G, Moldenaers P, Thomas S (2007) Cure kinetics, morphology, and miscibility of modified DGEBA-based epoxy resin: effects of a liquid rubber inclusion. Polymer 48:1695–1710
Thomas R, Yumei D, Yuelong H, Le Y, Moldenaers P, Weimin Y, Czigany T, Thomas S (2008) Miscibility, morphology, thermal and mechanical properties of a DGEBA based epoxy resin toughened with a liquid rubber. Polymer 49:278–294
Calabrese L, Valenza A (2003) Effect of CTBN rubber inclusions on the curing kinetic of DGEBA-DGEBF epoxy resin. Eur Polym J 39:1355–1363
ImageJ®. Software developed by the National Institutes of Health, USA
Castillo-Perez R, Romo-Uribe A (2012) Diseño y construcción de un instrumento para medir ángulo de contacto. Memorias del XVIII Congreso Internacional Anual de la SOMIM, ISBN: 978-607-95309-6-9, pp 772–779
Flores A, Ania F, Balta-Calleja FJ (2009) From the glassy state to ordered polymer structures. A microhardness study. Polymer 50:729–746
Ferry JD (1980) Viscoelastic properties of polymers. Wiley, New York
Cañamero-Martínez P, Fernández-García M, de la Fuente JL (2010) Rheological cure characterization of a polyfunctional epoxy acrylic resin. React Funct Polym 70:761–766
Wisanrakkit G, Gillham JK (1990) The glass transition temperature (T g ) as an index of chemical conversion for a high-T g amine/epoxy system: chemical and diffusion-controlled reaction kinetics. J Appl Polym Sci 41:2885–2929
Mortimer S, Ryan AJ, Stanford JL (2001) Rheological behavior and gel-point determination for a model lewis acid-initiated chain growth epoxy resin. Macromolecules 34:2973–2980
Takiguchi O, Ishikawa D, Sugimoto M, Taniguchi T, Koyama K (2008) Effect of rheological behavior of epoxy during procuring on foaming. J Appl Polym Sci 110:657–662
Choe Y, Kim M, Kim W (2003) In situ detection of the onset of phase separation and gelation in epoxy/anhydride/thermoplastic blends. Macromol Res 11:267–272
Neumann AW, Good RJ, Hope CJ, Sejpal M (1974) An equation-of-state approach to determine surface tensions of low-energy solids from contact angles. J Colloid Interface Sci 4:291–304
Spelt JK, Li D, Neumann AW (1992) Modern approaches to wettability. In: Schrader ME, Loeb GI (eds) Plenum Press, New York, pp 101–142
Grundke K, Bogumil T, Werner C, Janke A, Pöschel K, Jacobasch H (1996) Liquid-fluid contact angle measurements on hydrophilic cellulosic materials. J Colloid Surf A Physicochem Eng Asp 116:79–91
Güleç HA, Sarioglu K, Mutlu M (2006) Modification of food contacting surfaces by plasma polymerization technique. Part I: determination of hydrophilicity, hydrophobicity and surface free energy by contact angle method. J Food Eng 75:187–195
Acknowledgments
C. Valerio-Cardenas was supported by a postdoctoral fellowship from DGAPA–UNAM. This research was financially supported by PAPIIT (IG101313) and CONACyT, program CB-2011 (Grant 168095). A. E. G. is grateful to DGAPA-UNAM (PAPIIT Project IN-106008) for partial support. One of the authors (AF) wishes to thank the MICINN (Ministerio de Ciencia e Innovación), Spain, for financial support of the research (Grant FIS2010-18069).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Romo-Uribe, A., Arcos-Casarrubias, J.A., Flores, A. et al. Influence of rubber on the curing kinetics of DGEBA epoxy and the effect on the morphology and hardness of the composites. Polym. Bull. 71, 1241–1262 (2014). https://doi.org/10.1007/s00289-014-1121-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00289-014-1121-6