Abstract
Purpose
The rationale of the current study was to develop 6-mercaptopurine (6-MP)-mediated hematological toxicity prediction model for acute lymphoblastic leukemia (ALL) therapeutic management.
Methods
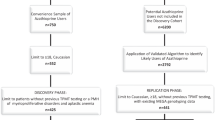
A total of 96 children with ALL undergoing therapy with MCP-841 protocol were screened for all the ten exons of TPMT, exon 2, exon 3 and intron 2 of ITPA using bidirectional sequencing. This dataset was used to construct prediction models of leucopenia grade by constructing classification and regression trees (CART) followed by smart pruning.
Results
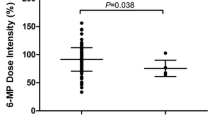
The developed CART model indicated TPMT*12 and TPMT*3C as the key determinants of toxicity. TPMT int3, int4 and int7 polymorphisms exert toxicity when co-segregated with one mutated allele of TPMT*12 or TPMT*3C or ITPA exon 3. The developed CART model exhibited 93.6% accuracy in predicting the toxicity. The area under the receiver operating characteristic curve was 0.9649.
Conclusions
TPMT *3C and TPMT*12 are the key determinants of 6-MP-mediated hematological toxicity while other variants of TPMT (int3, int4 and int7) and ITPA ex2 interact synergistically with TPMT*3C or TPMT*12 variant alleles to enhance the toxicity. TPMT and ITPA variants cumulatively are excellent predictors of 6-MP-mediated toxicity.


Similar content being viewed by others
Data availability
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Gerbek T, Ebbesen M, Nersting J, Frandsen TL, Appell ML, Schmiegelow K (2018) Role of TPMT and ITPA variants in mercaptopurine disposition. Cancer Chemother Pharmacol 81(3):579–586
El-Rashedy FH, Ragab SM, Dawood AA, Temraz SA (2015) Clinical implication of thiopurine methyltransferase polymorphism in children with acute lymphoblastic leukemia: a preliminary Egyptian study. Indian J Med Paediatr Oncol 36(4):265–270
Chrzanowska M, Kuehn M, Januszkiewicz-Lewandowska D, Kurzawski M, Droździk M (2012) Thiopurine S-methyltransferase phenotype-genotype correlation in children with acute lymphoblastic leukemia. Acta Pol Pharm 69(3):405–410
Adam de Beaumais T, Fakhoury M, Medard Y, Azougagh S, Zhang D, Yakouben K, Jacqz-Aigrain E (2011) Determinants of mercaptopurine toxicity in paediatric acute lymphoblastic leukemia maintenance therapy. Br J Clin Pharmacol 71(4):575–584
Dorababu P, Nagesh N, Linga VG, Gundeti S, Kutala VK, Reddanna P, Digumarti R (2012) Epistatic interactions between thiopurine methyltransferase (TPMT) and inosine triphosphate pyrophosphatase (ITPA) variations determine 6-mercaptopurine toxicity in Indian children with acute lymphoblastic leukemia. Eur J Clin Pharmacol 68(4):379–387
Dorababu P, Naushad SM, Linga VG, Gundeti S, Nagesh N, Kutala VK, Reddanna P, Digumarti R (2012) Genetic variants of thiopurine and folate metabolic pathways determine 6-MP-mediated hematological toxicity in childhood ALL. Pharmacogenomics 13(9):1001–1008
Ogungbenro K, Aarons L, CRESim, Epi-CRESim Project Groups (2015) Physiologically based pharmacokinetic model for 6-mercaptopurine: exploring the role of genetic polymorphism in TPMT enzyme activity. Br J Clin Pharmacol 80(1):86–100
Waljee AK, Joyce JC, Wang S, Saxena A, Hart M, Zhu J, Higgins PD (2010) Algorithms outperform metabolite tests in predicting response of patients with inflammatory bowel disease to thiopurines. Clin Gastroenterol Hepatol 8(2):143–150
Ma XL, Zhu P, Wu MY, Li ZG, Hu YM (2003) Relationship between single nucleotide polymorphisms in thiopurine methyltransferase gene and tolerance to thiopurines in acute leukemia. Zhonghua Er Ke Za Zhi 41(12):929–933
Hamdan-Khalil R, Allorge D, Lo-Guidice JM, Cauffiez C, Chevalier D, Spire C, Houdret N, Libersa C, Lhermitte M, Colombel JF, Gala JL, Broly F (2003) In vitro characterization of four novel non-functional variants of the thiopurine S-methyltransferase. Biochem Biophys Res Commun 309(4):1005–1010
Lee MN, Kang B, Choi SY, Kim MJ, Woo SY, Kim JW, Choe YH, Lee SY (2015) Impact of genetic polymorphisms on 6-thioguanine nucleotide levels and toxicity in pediatric patients with IBD treated with azathioprine. Inflamm Bowel Dis 21(12):2897–2908
Yi ES, Choi YB, Choi R, Lee NH, Lee JW, Yoo KH, Sung KW, Lee SY, Koo HH (2018) NUDT15 variants cause hematopoietic toxicity with low 6-TGN levels in children with acute lymphoblastic leukemia. Cancer Res Treat 50(3):872–882
Tanaka Y (2017) Susceptibility to 6-mercaptopurine toxicity related with NUDT15 and ABCC4 variants in Japanese childhood acute lymphoblastic leukemia. Rinsho Ketsueki 58(8):950–956
Smid A, Karas-Kuzelicki N, Jazbec J, Mlinaric-Rascan I (2016) PACSIN2 polymorphism is associated with thiopurine-induced hematological toxicity in children with acute lymphoblastic leukaemia undergoing maintenance therapy. Sci Rep 6:30244
Acknowledgements
We acknowledge the team of Department of Medical Oncology, Nizam’s Institute of Medical Sciences, Hyderabad for their cooperation for this study.
Funding
No funding was received for this work.
Author information
Authors and Affiliations
Contributions
The study was conceived and designed by DR and VKK. The research and analytical work was carried out by SMN, PD, SAA and TH. The statistical models were developed and manuscript was drafted by SMN and VKK.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Naushad, S.M., Dorababu, P., Rupasree, Y. et al. Classification and regression tree-based prediction of 6-mercaptopurine-induced leucopenia grades in children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol 83, 875–880 (2019). https://doi.org/10.1007/s00280-019-03803-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-019-03803-8