Abstract
Purpose
This population pharmacokinetic model was developed to characterize the pharmacokinetics of the oral irreversible ErbB family blocker afatinib in patients with solid tumors and to investigate the impact of selected intrinsic and extrinsic factors.
Methods
Data from 927 patients (4,460 plasma concentrations) with advanced solid tumors in 7 Phase II or III studies were analyzed. Afatinib was administered orally in continuous 3 or 4 week cycles (starting dose 20, 40 or 50 mg once-daily). Plasma concentration–time data for up to 7 months dosing were analyzed using nonlinear mixed-effects modeling.
Results
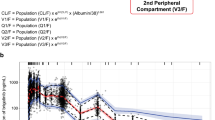
The pharmacokinetic profile of afatinib was best described by a two-compartment disposition model with first-order absorption and linear elimination. There was a slightly more than proportional increase in exposure with increasing dose, accounted for by a dose-dependent relative bioavailability. For the therapeutic dose of 40 mg, the estimated apparent total clearance and distribution volume at steady state were 734 mL/min and 2,370 L, respectively. While food intake, body weight, gender, Eastern Cooperative Oncology Group performance score, renal function, and the level of alkaline phosphatase, lactate dehydrogenase or total protein were statistically significant covariates influencing afatinib exposure, none resulted in a proportional change in exposure of more than 27.8 % in a typical patient at model extremes (2.5th and 97.5th percentiles of baseline values for continuous covariates). In simulations of the individual covariate effects, none caused a change in the typical profile exceeding the observed variability range (90 % prediction interval) of afatinib.
Conclusion
This population pharmacokinetic model adequately described the pharmacokinetics of afatinib in different cancer patient populations and therefore can be used for simulations exploring covariate effects and possible dose adaptations. The effect size for each of the individual covariates is not considered clinically relevant.



Similar content being viewed by others
References
Solca F, Dahl G, Zoephel A, Bader G, Sanderson M, Klein C, Kraemer O, Himmelsbach F, Haaksma E, Adolf GR (2012) Target binding properties and cellular activity of afatinib (BIBW 2992), an irreversible ErbB family blocker. J Pharmacol Exp Ther 343(2):342–350
Lin NU, Winer EP, Wheatley D, Carey LA, Houston S, Mendelson D, Munster P, Frakes L, Kelly S, Garcia AA, Cleator S, Uttenreuther-Fischer M, Jones H, Wind S, Vinisko R, Hickish T (2012) A phase II study of afatinib (BIBW 2992), an irreversible ErbB family blocker, in patients with HER2-positive metastatic breast cancer progressing after trastuzumab. Breast Cancer Res Treat 133(3):1057–1065
Schuler M, Awada A, Harter P, Canon JL, Possinger K, Schmidt M, De Greve J, Neven P, Dirix L, Jonat W, Beckmann MW, Schutte J, Fasching PA, Gottschalk N, Besse-Hammer T, Fleischer F, Wind S, Uttenreuther-Fischer M, Piccart M, Harbeck N (2012) A phase II trial to assess efficacy and safety of afatinib in extensively pretreated patients with HER2-negative metastatic breast cancer. Breast Cancer Res Treat 134(3):1149–1159
Miller VA, Hirsh V, Cadranel J, Chen YM, Park K, Kim SW, Zhou C, Su WC, Wang M, Sun Y, Heo DS, Crino L, Tan EH, Chao TY, Shahidi M, Cong XJ, Lorence RM, Yang JC (2012) Afatinib versus placebo for patients with advanced, metastatic non-small-cell lung cancer after failure of erlotinib, gefitinib, or both, and one or two lines of chemotherapy (LUX-Lung 1): a phase 2b/3 randomised trial. Lancet Oncol 13(5):528–538
Yang JC, Shih JY, Su WC, Hsia TC, Tsai CM, Ou SH, Yu CJ, Chang GC, Ho CL, Sequist LV, Dudek AZ, Shahidi M, Cong XJ, Lorence RM, Yang PC, Miller VA (2012) Afatinib for patients with lung adenocarcinoma and epidermal growth factor receptor mutations (LUX-Lung 2): a phase 2 trial. Lancet Oncol 13(5):539–548
Sequist LV, Yang JC, Yamamoto N, O’Byrne K, Hirsh V, Mok T, Geater SL, Orlov S, Tsai CM, Boyer M, Su WC, Bennouna J, Kato T, Gorbunova V, Lee KH, Shah R, Massey D, Zazulina V, Shahidi M, Schuler M (2013) Phase III study of afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol 31(27):3327–3334
Murakami H, Tamura T, Takahashi T, Nokihara H, Naito T, Nakamura Y, Nishio K, Seki Y, Sarashina A, Shahidi M, Yamamoto N (2012) Phase I study of continuous afatinib (BIBW 2992) in patients with advanced non-small cell lung cancer after prior chemotherapy/erlotinib/gefitinib (LUX-Lung 4). Cancer Chemother Pharmacol 69(4):891–899
Katakami N, Atagi S, Goto K, Hida T, Horai T, Inoue A, Ichinose Y, Koboyashi K, Takeda K, Kiura K, Nishio K, Seki Y, Ebisawa R, Shahidi M, Yamamoto N (2013) LUX-Lung 4: a phase II trial of afatinib in patients with advanced non-small-cell lung cancer who progressed during prior treatment with erlotinib, gefitinib, or both. J Clin Oncol 31(27):3335–3341
Cohen EEW, Fayette J, Cupissol D, Del Campo JM, Clement PM, Tourani JM, Degardin M, Zhang W, Ehrnrooth E, Seiwert TY (2012) A randomized, open-label, phase II study of afatinib (BIBW 2992) versus cetuximab in recurrent or metastatic squamous cell carcinoma of the head and neck: final data (abstract PP101). Eur Arch Otorhinolaryngol 269(4):1374
Wind S, Schmid M, Erhardt J, Goeldner RG, Stopfer P (2013) Pharmacokinetics of afatinib, a selective irreversible ErbB family blocker, in patients with advanced solid tumours. Clin Pharmacokinet 52(12):1101–1109
Yap TA, Vidal L, Adam J, Stephens P, Spicer J, Shaw H, Ang J, Temple G, Bell S, Shahidi M, Uttenreuther-Fischer M, Stopfer P, Futreal A, Calvert H, de Bono JS, Plummer R (2010) Phase I trial of the irreversible EGFR and HER2 kinase inhibitor BIBW 2992 in patients with advanced solid tumors. J Clin Oncol 28(25):3965–3972
Stopfer P, Marzin K, Narjes H, Gansser D, Shahidi M, Uttereuther-Fischer M, Ebner T (2012) Afatinib pharmacokinetics and metabolism after oral administration to healthy male volunteers. Cancer Chemother Pharmacol 69(4):1051–1061
Wind S, Giessmann T, Jungnik A, Brand T, Marzin K, Bertulis J, Hocke J, Gansser D, Stopfer P (2014) Pharmacokinetic drug interactions of afatinib with rifampicin and ritonavir. Clin Drug Investig Jan 8 (Epub ahead of print)
Eskens FA, Mom CH, Planting AS, Gietema JA, Amelsberg A, Huisman H, van Doorn L, Burger H, Stopfer P, Verweij J, de Vries EG (2008) A phase I dose escalation study of BIBW 2992, an irreversible dual inhibitor of epidermal growth factor receptor 1 (EGFR) and 2 (HER2) tyrosine kinase in a 2-week on, 2-week off schedule in patients with advanced solid tumours. Br J Cancer 98(1):80–85
Lindbom L, Ribbing J, Jonsson EN (2004) Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75(2):85–94
Lindbom L, Pihlgren P, Jonsson EN (2005) PsN-Toolkit—a collection of computer intensive statistical methods for non-linear mixed effect modeling using NONMEM. Comput Methods Programs Biomed 79(3):241–257
Food and Drug Administration Center for Drug Evaluation and Research (2013). Afatinib clinical pharmacology NDA review, Nov 2013. http://www.accessdata.fda.gov/drugsatfda_docs/nda/2013/201292Orig1s000ClinPharmR.pdf. Accessed 21 Nov 2013
Cockcroft DW, Gault MH (1976) Prediction of creatinine clearance from serum creatinine. Nephron 16(1):31–41
Yardley DA (2009) Proactive management of adverse events maintains the clinical benefit of ixabepilone. Oncologist 14(5):448–455
Bristol-Myers Squibb (2011). Ixempra kit (ixabepilone) for injection, for intravenous infusion only. Prescribing information, revised: 10/2011. http://packageinserts.bms.com/pi/pi_ixempra.pdf. Accessed 21 Nov 2013
Bergstrand M, Hooker AC, Wallin JE, Karlsson MO (2011) Prediction-corrected visual predictive checks for diagnosing nonlinear mixed-effects models. AAPS J 13(2):143–151
Food and Drug Administration (2010). Guidance for industry: pharmacokinetics in patients with impaired renal function—study design, data analysis, and impact on dosing and labeling. March 2010. http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM204959.pdf. Accessed 21 Nov 2013
Schnell D, Buschke S, Fuchs H, Göldner R-G, Uttenreuther-Fischer M, Stopfer P, Wind S, Halabi A, Koenen R (2012) Phase I study to compare safety and pharmacokinetics of afatinib, an oral irreversible ErbB family blocker, in non-cancer subjects with hepatic impairment to matched healthy subjects (abstract 468P). Ann Oncol 23(Suppl 9):162
Acknowledgments
These studies were sponsored by Boehringer Ingelheim Pharma GmbH & Co KG, Germany. Boehringer Ingelheim was responsible for the design and conduct of all of the studies, and the collection and management of the data. The authors were responsible for the analysis and interpretation of the data and the preparation of the manuscript. The authors thank the medical staff of Boehringer Ingelheim responsible for each study, the investigators of each study, along with Mehdi Shahidi (Boehringer Ingelheim), Victoria Zazulina (Boehringer Ingelheim), and Dietmar Gansser (Boehringer Ingelheim) and his staff, for conducting the bioanalytical portion of the studies.
Conflict of interest
All authors are employees of Boehringer Ingelheim Pharma GmbH & Co KG. The authors have no other conflict of interest directly related to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Freiwald, M., Schmid, U., Fleury, A. et al. Population pharmacokinetics of afatinib, an irreversible ErbB family blocker, in patients with various solid tumors. Cancer Chemother Pharmacol 73, 759–770 (2014). https://doi.org/10.1007/s00280-014-2403-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-014-2403-2