Abstract
Purpose
Histone deacetylase inhibitors (HDACIs), such as PXD101 and suberoylanilide hydroxamic acid, inhibit proliferation and stimulate apoptosis of tumor cells. The enhanced effectiveness of chemotherapy or radiotherapy when combined with HDACIs has been observed in several cancers. In this study, we investigated the antitumor effect of PXD101 combined with irinotecan in colon cancer.
Methods
HCT116 and HT29 colon cancer cells for cell viability assay were treated with PXD101 and/or SN-38, the active form of irinotecan. Antitumor effects of HCT116 and HT29 xenografts treated with these combinations were evaluated. [18F]FLT-PET was used to detect early responses to PXD101 and irinotecan in colon cancer.
Results
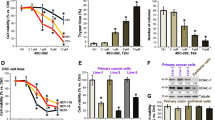
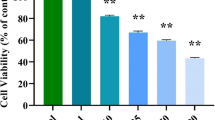
PXD101 and SN38 possessed dose-dependent antiproliferative activity against HCT116 and HT29 cells and exerted a synergistic effect when used in combination. In xenografted mice, PXD101 in combination with irinotecan dramatically inhibited tumor growth without causing additive toxicity. Apoptotic effects on xenograft tumors were greater with combined treatment than with irinotecan alone. [18F]FLT-PET imaging revealed a 64% decrease in [18F]FLT uptake in tumors of HCT116 xenograft-bearing mice treated with a combination of PXD101 and irinotecan, indicating a decrease in thymidine kinase 1 (TK1) activity. These results were supported by Western blot analyses showing a decrease in tumor thymidine kinase 1 protein levels, suggesting that [18F]FLT-PET can be used to non-invasively detect early responses to these agents.
Conclusions
These data show that PXD101 increases the cytotoxic activity of irinotecan in in vitro and in vivo colon cancer models and suggest these agent combinations should be explored in the treatment of colon cancer.






Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Hao Y, Xu J et al (2009) Cancer statistics, 2009. CA Cancer J Clin 59:225–249
Kohne CH, Lenz HJ (2009) Chemotherapy with targeted agents for the treatment of metastatic colorectal cancer. Oncologist 14:478–488
Weekes J, Lam AK, Sebesan S, Ho YH (2009) Irinotecan therapy and molecular targets in colorectal cancer: a systemic review. World J Gastroenterol 15:3597–3602
Teicher BA (2008) Next generation topoisomerase I inhibitors: rationale and biomarker strategies. Biochem Pharmacol 75:1262–1271
Marks PA, Xu WS (2009) Histone deacetylase inhibitors: potential in cancer therapy. J Cell Biochem 107:600–608
Knupfer MM, Pulzer F, Schindler I, Hernaiz Driever P, Knupfer H et al (2001) Different effects of valproic acid on proliferation and migration of malignant glioma cells in vitro. Anticancer Res 21:347–351
Witt O, Monkemeyer S, Kanbach K, Pekrun A (2002) Induction of fetal hemoglobin synthesis by valproate: modulation of MAP kinase pathways. Am J Hematol 71:45–46
Yuan PX, Huang LD, Jiang YM, Gutkind JS, Manji HK et al (2001) The mood stabilizer valproic acid activates mitogen-activated protein kinases and promotes neurite growth. J Biol Chem 276:31674–31683
Wilson AJ, Byun DS, Popova N, Murray LB, L’Italien K et al (2006) Histone deacetylase 3 (HDAC3) and other class I HDACs regulate colon cell maturation and p21 expression and are deregulated in human colon cancer. J Biol Chem 281:13548–13558
Nakagawa M, Oda Y, Eguchi T, Aishima S, Yao T et al (2007) Expression profile of class I histone deacetylases in human cancer tissues. Oncol Rep 18:769–774
Mariadason JM (2008) HDACs and HDAC inhibitors in colon cancer. Epigenetics 3:28–37
Nian H, Bisson WH, Dashwood WM, Pinto JT, Dashwood RH (2009) Alpha-keto acid metabolites of organoselenium compounds inhibit histone deacetylase activity in human colon cancer cells. Carcinogenesis 30:1416–1423
Buggy JJ, Cao ZA, Bass KE, Verner E, Balasubramanian S et al (2006) CRA-024781: a novel synthetic inhibitor of histone deacetylase enzymes with antitumor activity in vitro and in vivo. Mol Cancer Ther 5:1309–1317
Fazzone W, Wilson PM, Labonte MJ, Lenz HJ, Ladner RD (2009) Histone deacetylase inhibitors suppress thymidylate synthase gene expression and synergize with the fluoropyrimidines in colon cancer cells. Int J Cancer 125:463–473
Kim JC, Shin ES, Kim CW, Roh SA, Cho DH et al (2009) In vitro evaluation of histone deacetylase inhibitors as combination agents for colorectal cancer. Anticancer Res 29:3027–3034
Prince HM, Bishton MJ, Harrison SJ (2009) Clinical studies of histone deacetylase inhibitors. Clin Cancer Res 15:3958–3969
Stimson L, La Thangue NB (2009) Biomarkers for predicting clinical responses to HDAC inhibitors. Cancer Lett 280:177–183
Salskov A, Tammisetti VS, Grierson J, Vesselle H (2007) FLT: measuring tumor cell proliferation in vivo with positron emission tomography and 3′-deoxy-3′-[18F]fluorothymidine. Semin Nucl Med 37:429–439
Shields AF, Grierson JR, Dohmen BM, Machulla HJ, Stayanoff JC et al (1998) Imaging proliferation in vivo with [F-18]FLT and positron emission tomography. Nat Med 4:1334–1336
Rasey JS, Grierson JR, Wiens LW, Kolb PD, Schwartz JL (2002) Validation of FLT uptake as a measure of thymidine kinase-1 activity in A549 carcinoma cells. J Nucl Med 43:1210–1217
Chou TC, Talalay P (1984) Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul 22:27–55
Chou TC, Motzer RJ, Tong Y, Bosl GJ (1994) Computerized quantitation of synergism and antagonism of taxol, topotecan, and cisplatin against human teratocarcinoma cell growth: a rational approach to clinical protocol design. J Natl Cancer Inst 86:1517–1524
Martin SJ, Eisenbarth JA, Wagner-Utermann U, Mier W, Henze M et al (2002) A new precursor for the radiosynthesis of [18F]FLT. Nucl Med Biol 29:263–273
Plumb JA, Finn PW, Williams RJ, Bandara MJ, Romero MR et al (2003) Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther 2:721–728
Niculescu AB 3rd, Chen X, Smeets M, Hengst L, Prives C et al (1998) Effects of p21(Cip1/Waf1) at both the G1/S and the G2/M cell cycle transitions: pRb is a critical determinant in blocking DNA replication and in preventing endoreduplication. Mol Cell Biol 18:629–643
Rodriguez R, Hansen LT, Phear G, Scorah J, Spang-Thomsen M et al (2008) Thymidine selectively enhances growth suppressive effects of camptothecin/irinotecan in MSI+cells and tumors containing a mutation of MRE11. Clin Cancer Res 14:5476–5483
Liu Y, **ng H, Weng D, Song X, Qin X et al (2009) Inhibition of Akt signaling by SN-38 induces apoptosis in cervical cancer. Cancer Lett 274:47–53
Dai Y, Chen S, Kramer LB, Funk VL, Dent P et al (2008) Interactions between bortezomib and romidepsin and belinostat in chronic lymphocytic leukemia cells. Clin Cancer Res 14:549–558
Leyton J, Alao JP, Da Costa M, Stavropoulou AV, Latigo JR et al (2006) In vivo biological activity of the histone deacetylase inhibitor LAQ824 is detectable with 3′-deoxy-3′-[18F]fluorothymidine positron emission tomography. Cancer Res 66:7621–7629
Voeller DM, Grem JL, Pommier Y, Paull K, Allegra CJ (2000) Identification and proposed mechanism of action of thymidine kinase inhibition associated with cellular exposure to camptothecin analogs. Cancer Chemother Pharmacol 45:409–416
Piacentini P, Donadelli M, Costanzo C, Moore PS, Palmieri M et al (2006) Trichostatin A enhances the response of chemotherapeutic agents in inhibiting pancreatic cancer cell proliferation. Virchows Arch 448:797–804
Zhang X, Yashiro M, Ren J, Hirakawa K (2006) Histone deacetylase inhibitor, trichostatin A, increases the chemosensitivity of anticancer drugs in gastric cancer cell lines. Oncol Rep 16:563–568
Marchion DC, Bicaku E, Daud AI, Richon V, Sullivan DM et al (2004) Sequence-specific potentiation of topoisomerase II inhibitors by the histone deacetylase inhibitor suberoylanilide hydroxamic acid. J Cell Biochem 92:223–237
Marchion DC, Bicaku E, Turner JG, Daud AI, Sullivan DM et al (2005) Synergistic interaction between histone deacetylase and topoisomerase II inhibitors is mediated through topoisomerase IIbeta. Clin Cancer Res 11:8467–8475
Fetterly GJ, Brady WE, LeVea CM, Litwin AM, Zagst PD, Prey JD, Tarquini M, Giardina MK, Iyer RV, Khushalani NI (2009) A phase I pharmacokinetic (PK) study of vorinostat in combination with irinotecan, 5-fluorouracil, and leucovorin in advanced upper gastrointestinal cancers. J Clin Oncol 27:15s (suppl; abstr e15540)
Takemura H, Rao VA, Sordet O, Furuta T, Miao ZH et al (2006) Defective Mre11-dependent activation of Chk2 by ataxia telangiectasia mutated in colorectal carcinoma cells in response to replication-dependent DNA double strand breaks. J Biol Chem 281:30814–30823
Glaser KB (2007) HDAC inhibitors: clinical update and mechanism-based potential. Biochem Pharmacol 74:659–671
LaBonte MJ, Wilson PM, Fazzone W, Groshen S, Lenz HJ et al (2009) DNA microarray profiling of genes differentially regulated by the histone deacetylase inhibitors vorinostat and LBH589 in colon cancer cell lines. BMC Med Genomics 2:67
Tredan O, Galmarini CM, Patel K, Tannock IF (2007) Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst 99:1441–1454
Jeon HW, Lee YM (2010) Inhibition of histone deacetylase attenuates hypoxia-induced migration and invasion of cancer cells via the restoration of RECK expression. Mol Cancer Ther 9:1361–1370
Shen J, Hughes C, Chao C, Cai J, Bartels C et al (1987) Coinduction of glucose-regulated proteins and doxorubicin resistance in Chinese hamster cells. Proc Natl Acad Sci USA 84:3278–3282
Gerweck LE, Vijayappa S, Kozin S (2006) Tumor pH controls the in vivo efficacy of weak acid and base chemotherapeutics. Mol Cancer Ther 5:1275–1279
Raghunand N, He X, van Sluis R, Mahoney B, Baggett B et al (1999) Enhancement of chemotherapy by manipulation of tumour pH. Br J Cancer 80:1005–1011
Mercalli A, Sordi V, Formicola R, Dandrea M, Beghelli S et al (2007) A preclinical evaluation of pemetrexed and irinotecan combination as second-line chemotherapy in pancreatic cancer. Br J Cancer 96:1358–1367
Acknowledgments
This study was supported by grants from the Korea Health 21 R&D Project, Ministry of Health, Welfare, and Family Affairs, Republic of Korea (A062254).
Conflicts of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Na, YS., Jung, KA., Kim, SM. et al. The histone deacetylase inhibitor PXD101 increases the efficacy of irinotecan in in vitro and in vivo colon cancer models. Cancer Chemother Pharmacol 68, 389–398 (2011). https://doi.org/10.1007/s00280-010-1495-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-010-1495-6