Abstract
Purpose
SPI-077 and SPI-077 B103 are formulations of cisplatin encapsulated in pegylated STEALTH liposomes that accumulate in tumors. However, the extent to which active platinum (Pt) is released from the liposome is unknown. Thus, we evaluated the disposition of encapsulated and released Pt in plasma and tumors after administration of STEALTH liposomal and nonliposomal cisplatin.
Methods
Cisplatin (10 mg/kg), SPI-077 (10 mg/kg), and SPI-077 B103 (5 mg/kg) were administered i.v. to mice bearing B16 murine melanoma tumors. Microdialysis probes were placed into the right and left sides of each tumor, and serial samples were collected from tumor extracellular fluid (ECF) after administration of each agent. After each microdialysis procedure, tumor samples were obtained at each probe site to measure total Pt and Pt-DNA adducts. In a separate study, serial plasma samples (three mice per time point) were obtained. Unbound Pt in tumor ECF and plasma, and total Pt in tumor homogenates were measured by flameless atomic absorption spectrophotometry. Area under the tumor ECF (AUCECF) concentration versus time curves of unbound Pt were calculated. Intrastrand GG (Pt-GG) and AG (Pt-AG) Pt-DNA adducts were measured via 32P-postlabeling.
Results
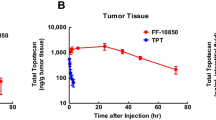
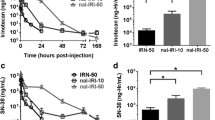
Mean±SD peak concentrations of total Pt in tumor homogenates after administration of cisplatin, SPI-077, and SPI-077 B-103 were 3.2±1.9, 11.9±3.0, and 3.5±0.3 μg/g, respectively. After cisplatin, mean±SD AUCECF of unbound Pt was 0.72±0.46 μg/ml·h. There was no detectable unbound Pt in tumor ECF after SPI-077 or SPI-077 B-103 treatment. Mean±SD peak concentration of Pt-GG DNA adducts after administration of cisplatin, SPI-077, and SPI-077 B-103 were 13.1±3.3, 3.5±1.3, and 2.1±0.3 fmol Pt/μg DNA, respectively.
Conclusion
This study suggests that more SPI-077 and SPI-077 B103 distribute into tumors, but release less Pt into tumor ECF, and form fewer Pt-DNA adducts than does cisplatin.

Similar content being viewed by others
References
Dorr RT, Von Hoff DD (1993) Cisplatin monograph, 2nd ed. In: Dorr RT, Von Hoff DD (eds) Cancer chemotherapy handbook. Appleton and Lange, New York, p 286
Harrington KJ, Lewanski CR, Northcote AD, Whittaker J, Wellbank H, Vile RG, Peters AM, Stewart JS (2001) Phase I-II study of pegylated liposomal cisplatin (SPI-077) in patients with inoperable head and neck cancer. Ann Oncol 12:493
DeMario MD, Vogelzang NJ, Janisch L, Tonda M, Amantes MA, Pendyala L, Ratain MJ (1998) A phase I study of liposome-formulated cisplatin (SPI-077) given every 3 weeks in patients with advanced cancer. Proc Am Soc Clin Oncol 17:883
Veal GJ, Griffin MJ, Price E, Parry A, Dick GS, Little MA, Yule SM, Morland B, Estlin EJ, Hale JP, Pearson AD, Welbank H, Boddy AV (2001) A phase I study in paediatric patients to evaluate the safety and pharmacokinetics of SPI-77, a liposome encapsulated formulation of cisplatin. Br J Cancer 84:1029
Schellens JHM, Meerum Terwogt J, Groenewegen G, Blijham GH, Ten Bokkel Huinink WW, Swart M, Maliepaard M, Floot B, Welbank H, Beijnen JH (1998) Phase I and pharmacologic study of SPI-77, a novel STEALTH liposomal encapsulated formulation of cisplatin (CDDP). Proc Am Assoc Cancer Res 39:2218
Newman MS, Colbern GT, Working PK, Engbers C, Amantea MA (1999) Comparative pharmacokinetics, tissue distribution, and therapeutic effectiveness of cisplatin encapsulated in long-circulating, pegylated liposomes (SPI-077) in tumor-bearing mice. Cancer Chemother Pharmacol 43:1
Gerad H, Egorin MJ, Whitacre M, Van Echo DA, Aisner J (1983) Renal failure and platinum pharmacokinetics in three patients treated with cis-diamminedichloroplatinum(II) and whole-body hyperthermia. Cancer Chemother Pharmacol 11:162
Egorin MJ, Van Echo DA, Tip** SJ, Olman EA, Whitacre MY, Thompson BW, Aisner J (1984) Pharmacokinetics and dosage reduction of cis-diammine(1,1-cyclobutanedicarboxylato)platinum in patients with impaired renal function. Cancer Res 44:5432
Allen TM, Stuart DD (1998) Liposomal pharmacokinetics. Classical, sterically-stabilized, cationic liposomes and immunoliposomes. In: Janoff AS (ed) Liposomes: rational design. Marcel Dekker, New York, p 63
Woodle MC, Lasic DD (1992) Sterically stabilized liposomes. Biochim Biophys Acta 1113:171
Pluim D, Maliepaard M, van Waardenburg RC, Beijnen JH, Schellens JH (1999) 32P-postlabeling assay for the quantification of the major platinum-DNA adducts. Anal Biochem 275:30
Reed E, Saurhoff S, Poirier MC (1998) Quantitation of platinum-DNA binding after therapeutic levels of drug exposure: a novel use of graphite furnace spectrometry. Atom Spectrosc 3:93
Johansen MJ, Newman RA, Madden T (1997) The use of microdialysis in pharmacokinetics and pharmacodynamics. Pharmacotherapy 17:464
Kehr J (1993) A survey on quantitative microdialysis: theoretical models and practical implications. J Neurosci Methods 48:251
Zamboni WC, Houghton PJ, Hulstein JL, Kirstein M, Walsh J, Cheshire PJ, Hanna SK, Danks MK, Stewart CF (1999) Relationship between tumor extracellular fluid exposure to topotecan and tumor response in human neuroblastoma xenograft and cell lines. Cancer Chemother Pharmacol 43:269
Muller M, Mader RM, Steiner B, Steger GG, Jansen B, Gnant M, Helbich T, Jakesz R, Eichler HG, Blochl-Daum B (1997) 5-Fluorouracil kinetics in the interstitial tumor space: clinical response in breast cancer patients. Cancer Res 57:2598
Blochl-Daum B, Muller M, Meisinger V, Eichler HG, Fassolt A, Pehamberger H (1996) Measurement of extracellular fluid carboplatin kinetics in melanoma metastases with microdialysis. Br J Cancer 73:920
Muller M, Schmid R, Georgopoulos A, Buxbaum A, Wasicek C, Eichler HG (1995) Application of microdialysis to clinical pharmacokinetics in humans. Clin Pharmacol Ther 57:371
Zamboni WC, Gervais AC, Egorin MJ, Schellens JH, Hamburger DR, Delauter BJ, Grim A, Zuhowski EG, Joseph E, Pluim D, Potter DM, Eiseman JL (2002) Inter- and intratumoral disposition of platinum in solid tumors after administration of cisplatin. Clin Cancer Res 8:2992
Conley BA, Ramsland TS, Sentz DL, Wu S, Rosen DM, Wollman M, Eiseman JL (1999) Antitumor activity, distribution, and metabolism of 13-cis-retinoic acid as a single agent or in combination with tamoxifen in established human MCF-7 xenografts in mice. Cancer Chemother Pharmacol 43:183
D’Argenio DZ, Schumitzky A (1979) A program package for simulation and parameter estimation in pharmacokinetic systems. Comput Programs Biomed 9:115
Zamboni WC, Luftner DI, Egorin MJ, Schweigert M, Sezer O, Richter T, Natale JJ, Possinger K (2001) The effect of increasing topotecan infusion from 30 minutes to 4 hours on the duration of exposure in cerebrospinal fluid. Ann Oncol 12:119
Gibaldi M, Perrier D (1982) Pharmacokinetics, 2nd edn. Marcel Dekker, New York
Hirsch RP, Riegelman RK (1992) Statistical first aid: interpretation of health research data. Blackwell Scientific, Boston
Rosner B (2000) Fundamentals of biostatistics, 5th edn. Duxbury, Pacific Grove, CA
Harrington KJ, Mohammadtaghi S, Uster PS, Glass D, Peters AM, Vile RG, Stewart JS (2001) Effective targeting of solid tumors in patients with locally advanced cancers by radiolabeled pegylated liposomes. Clin Cancer Res 7:243
Harrington KJ, Rowlinson-Busza G, Syrigos KN, Uster PS, Abra RM, Stewart JS (2000) Biodistribution and pharmacokinetics of 111In-DTPA-labelled pegylated liposomes in a human tumour xenograft model: implications for novel targeting strategies. Br J Cancer 83:232
Harrington KJ, Rowlinson-Busza G, Syrigos KN, Abra RM, Uster PS, Peters AM, Stewart JS (2000) Influence of tumour size on uptake of (111)ln-DTPA-labelled pegylated liposomes in a human tumour xenograft model. Br J Cancer 83:684
Northfelt DW (1994) STEALTH liposomal doxorubicin (SLD) delivers more DOX to AIDS-Kaposi’s sarcoma lesions than to normal skin. Proc Am Soc Clin Oncol 13:51
Stewart S, Jablonowski H, Goebel FD, Arasteh K, Spittle M, Rios A, Aboulafia D, Galleshaw J, Dezube BJ (1998) Randomized comparative trial of pegylated liposomal doxorubicin versus bleomycin and vincristine in the treatment of AIDS-related Kaposi’s sarcoma. International Pegylated Liposomal Doxorubicin Study Group. J Clin Oncol 16:683
Northfelt DW, Dezube BJ, Thommes JA, Miller BJ, Fischl MA, Friedman-Kien A, Kaplan LD, Du MC, Mamelok RD, Henry DH (1998) Pegylated-liposomal doxorubicin versus doxorubicin, bleomycin, and vincristine in the treatment of AIDS-related Kaposi’s sarcoma: results of a randomized phase III clinical trial. J Clin Oncol 16:2445
Muggia FM, Hainsworth JD, Jeffers S, Miller P, Groshen S, Tan M, Roman L, Uziely B, Muderspach L, Garcia A, Burnett A, Greco FA, Morrow CP, Paradiso LJ, Liang LJ (1997) Phase II study of liposomal doxorubicin in refractory ovarian cancer: antitumor activity and toxicity modification by liposomal encapsulation. J Clin Oncol 15:987
Berry G, Billingham M, Alderman E, Richardson P, Torti F, Lum B, Patek A, Martin FJ (1998) The use of cardiac biopsy to demonstrate reduced cardiotoxicity in AIDS Kaposi’s sarcoma patients treated with pegylated liposomal doxorubicin. Ann Oncol 9:711
Peleg-Shulman T, Gibson D, Cohen R, Abra R, Barenholz Y (2001) Characterization of sterically stabilized cisplatin liposomes by nuclear magnetic resonance. Biochim Biophys Acta 1510:278
Colbern GT, Dykes DJ, Engbers C, Musterer R, Hiller A, Pegg E, Saville R, Weng S, Luzzio M, Uster P, Amantea M, Working PK (1998) Encapsulation of the topoisomerase I inhibitor GL147211C in pegylated (STEALTH) liposomes: pharmacokinetics and antitumor activity in HT29 colon tumor xenografts. Clin Cancer Res 4:3077
Allen TM, Hansen C (1991) Pharmacokinetics of stealth versus conventional liposomes: effect of dose. Biochim Biophys Acta 1068:133
Papahadjopoulos D, Allen TM, Gabizon A, Mayhew E, Matthay K, Huang SK, Lee KD, Woodle MC, Lasic DD, Redemann C (1991) Sterically stabilized liposomes: improvements in pharmacokinetics and antitumor therapeutic efficacy. Proc Natl Acad Sci U S A 88:11460
Drumond DC, Meyer O, Hong K (1991) Optimizing liposomes for delivery of chemotherapeutic agents to solid tumor. Pharmacol Rev 51:692
Working PK, Newman MS, Huang SK (1994) Pharmacokinetics, biodistribution, and therapeutic efficacy of doxorubicin encapsulated in STEALTH liposomes. Liposome Res 46:667
Jain RK (1996) Delivery of molecular medicine to solid tumors. Science 271:1079
Proulx ME, Desormeaux A, Marquis JF, Olivier M, Bergeron MG (2001) Treatment of visceral leishmaniasis with sterically stabilized liposomes containing camptothecin. Antimicrob Agents Chemother 45:2623
Tardi P, Choice E, Masin D, Redelmeier T, Bally M, Madden TD (2000) Liposomal encapsulation of topotecan enhances anticancer efficacy in murine and human xenograft models. Cancer Res 60:3389
Verschraegen CF, Gilbert BE, Huaringa AJ, Newman R, Harris N, Leyva FJ, Keus L, Campbell K, Nelson-Taylor T, Knight V (2000) Feasibility, phase I, and pharmacologic study of aerosolized 9-nitro-20(S)-camptothecin in patients with advanced malignancies in the lungs. Ann N Y Acad Sci 922:352
Desjardins JP, Abbott EA, Emerson DL, Tomkinson BE, Leray JD, Brown EN, Hamilton M, Dihel L, Ptaszynski M, Bendele RA, Richardson FC (2001) Biodistribution of NX211, liposomal lurtotecan, in tumor bearing mice. Anticancer Drugs 12:235
Sharma A, Straubinger RM, Ojima I, Bernacki RJ (1995) Antitumor efficacy of taxane liposomes on a human ovarian tumor xenograft in nude athymic mice. J Pharm Sci 84:1400
Ducan R, Gac-Breton S, Keane R, Musila R, Sat YN, Satchi R, Searle F (2001) Polymer-drug conjugates, PDEPT and PELT: basic principles for design and transfer from laboratory to clinic. J Control Release 74:135
Sharma A, Straubinger RM, Ojima I, Bernacki RJ (1995) Antitumor efficacy of taxane liposomes on a human ovarian tumor xenograft in nude athymic mice. J Pharm Sci 84:1400–1404
Ducan R, Gac-Breton S, Keane R, Musila R, Sat YN, Satchi R, Searle F (2000) Polymer drug conjugates, PDEPT and PELT: basic principles for design and transfer from laboratory to clinic. J Control Release 74:135–146
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zamboni, W.C., Gervais, A.C., Egorin, M.J. et al. Systemic and tumor disposition of platinum after administration of cisplatin or STEALTH liposomal-cisplatin formulations (SPI-077 and SPI-077 B103) in a preclinical tumor model of melanoma. Cancer Chemother Pharmacol 53, 329–336 (2004). https://doi.org/10.1007/s00280-003-0719-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00280-003-0719-4