Abstract
Background
Enhanced Recovery After Surgery (ERAS) has been widely applied in liver surgery since the publication of the first ERAS guidelines in 2016. The aim of the present article was to update the ERAS guidelines in liver surgery using a modified Delphi method based on a systematic review of the literature.
Methods
A systematic literature review was performed using MEDLINE/PubMed, Embase, and the Cochrane Library. A modified Delphi method including 15 international experts was used. Consensus was judged to be reached when >80% of the experts agreed on the recommended items. Recommendations were based on the Grading of Recommendations, Assessment, Development and Evaluations system.
Results
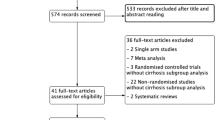
A total of 7541 manuscripts were screened, and 240 articles were finally included. Twenty-five recommendation items were elaborated. All of them obtained consensus (>80% agreement) after 3 Delphi rounds. Nine items (36%) had a high level of evidence and 16 (64%) a strong recommendation grade. Compared to the first ERAS guidelines published, 3 novel items were introduced: prehabilitation in high-risk patients, preoperative biliary drainage in cholestatic liver, and preoperative smoking and alcohol cessation at least 4 weeks before hepatectomy.
Conclusions
These guidelines based on the best available evidence allow standardization of the perioperative management of patients undergoing liver surgery. Specific studies on hepatectomy in cirrhotic patients following an ERAS program are still needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Enhanced Recovery After Surgery (ERAS) is a multimodal and perioperative management pathway. ERAS offers to reduce the response to surgical stress and has been shown to decrease postoperative complications and length of stay (LoS) after several types of surgery [1, 2].
The first ERAS guidelines for liver surgery were published in 2016 [3]. Since then, several publications have shown that implementation of ERAS in liver surgery improves postoperative outcomes [4, 5]. Three recent meta-analyses showed that ERAS in liver surgery decreased postoperative complications, LoS, and costs [6,207].
Summary and recommendation: Use of an omental flap to cover the cut surface of the liver might reduce the risk of delayed gastric emptying after left-sided liver resection.
Evidence level: Low.
Grade of recommendation: Weak.
Stimulation of bowel movement
Shimada et al. [208] found in a multicenter RCT that daikenchuto (TU-100, traditional herbal medicine) significantly decreased the median time to first bowel movement by 5 h in 231 patients who underwent hepatectomy for cancer. Although significant, this 5-h difference is probably not clinically relevant as complication rates were similar in both groups. Another RCT assessing the effect of daikenchuto after hepatectomy found that the daikenchuto group had shorter time to bowel movement and oral intake, but complications were similar [209]. You et al. [210] performed a 3-arm RCT to assess ileus rates in patients with HCC undergoing liver resection. Simo decoction (traditional Chinese herbal medicine) with acupuncture was compared to gum chewing and no specific postoperative intervention (control group). Both interventions were found to diminish the time to first stool compared to the control group, whereas only the group with simo decoction and acupuncture had a shorter length of hospital stay. In a RCT of 68 patients undergoing liver surgery, the group with laxatives had reduced time to passage of stool but similar secondary outcomes, such as DGE, LoS, or time to functional recovery [211]. Jang et al. [212] showed in a prospective case–control study including 42 patients that gum chewing permitted to decrease the time to first flatus and the xerostomia rate, but did not have an effect on LoS or analgesic use.
Summary and recommendation: Postoperative laxatives, gum chewing, herbal medicine, or decoction after hepatectomy might reduce the time to first flatus or stool but do not impact the morbidity rate. Current data do not permit the recommendation of the routine use of postoperative laxatives, gum chewing, herbal medicine, or decoction to stimulate bowel movement after liver surgery.
Evidence level: Moderate.
Grade of recommendation: Weak.
Early and scheduled mobilization
Bed rest is associated with multiple established deleterious effects including muscle atrophy, thromboembolic disease, and insulin resistance [213,214,215]. A RCT involving 120 patients undergoing liver resection showed a significantly faster postoperative gastrointestinal function and shorter length of hospital stay after performing early activity (from postoperative day 1) [216]. An early postoperative mobilization program based on supervised exercises improved functional capacity in patients undergoing major elective abdominal oncologic surgery [217].
So far, however, no consensus has been defined regarding the type, frequency, and intensity of physical therapy in liver surgery [218].
Summary and recommendation: Early mobilization (out of bed) after liver surgery should be established from the operative day until hospital discharge. No recommendation can be made regarding the optimal duration of mobilization.
Evidence level: Moderate.
Grade of recommendation: Strong.
Postoperative nausea and vomiting (PONV) prophylaxis
PONV occurs frequently after major surgery (25–30%). The multimodal approach and opioid reduction provided by ERAS enable the majority of patients to eat early after hepatectomy [219]. Known risk factors, such as previous PONV, female gender, younger age, non-smoker, and use of volatile anesthetic agents and opioids, should be evaluated before the operation [220]. The 5-HT3 antagonists are the primary treatment because of their safe side effect profile. Low-dose dexamethasone is a good additive preventative agent and facilitates hepatic regeneration [221]. Of note, there is no supplementary advantage of using higher doses [221]. However, dexamethasone should be used with caution in diabetics as it can transiently worsen glycemic control [222]. Antihistamines, butyrophenones, and phenothiazines can also be used as second-line therapy [88]. The international consensus group on PONV recommends using 2 antiemetic drugs to decrease PONV and to improve efficacy [88]. Table 3 summarizes potential antiemetic drugs with doses and timing of use.
Summary and recommendation: A multimodal approach to postoperative nausea and vomiting should be used. Patients should receive postoperative nausea and vomiting prophylaxis with at least 2 antiemetic drugs such as dexamethasone and ondansetron.
Evidence level: High.
Grade of recommendation: Strong.
Fluid management
Blood loss and transfusion rates remain central risk factors leading to higher morbidity and mortality after liver resections [223,224,225]. A Cochrane review showed that a lower central venous pressure (CVP) decreased blood loss, but without significant difference in red blood cell transfusion requirements, intraoperative morbidity, or long-term survival benefits [226]. Another systematic review and meta-analysis also confirmed that low CVP was associated with less blood loss [227].
Regarding fluid management for major hepatic surgery, there is currently no protocol available providing the optimum amount of fluid to be given to patients. The current concept must focus on the maintenance of central euvolemia, thereby preventing any excess of salt or water. Goal-directed fluid therapy (GDFT), targeting adequate cardiac output and end-organ perfusion, has attracted much attention. A meta-analysis of 32 RCT including about 3000 patients testing the impact of GDFT during major surgery, i.e., not only liver surgery, demonstrated significant benefits in reducing morbidity and mortality [228]. A RCT published in 2015 found that stroke volume variation (SVV)-guided GDFT compared to standard fluid resuscitation decreased the intraoperative infused fluid volume without decreasing postoperative complications [229]. On multivariable analysis, higher intraoperative fluid volume was an independent risk factor for 30-day morbidity. Recently, Weinberg et al. [230] showed in an RCT that a restrictive GDFT did not decrease LoS and fluid-related complications compared to conventional care within an ERAS pathway for major liver resection. Of note, only 24 patients were included in each arm.
Excessive administration of crystalloids should be avoided as much as blood loss during liver surgery. To guide fluid management during surgery, the measurement of SVV has been proposed to replace CVP monitoring [231]. A randomized prospective trial comparing SVV monitoring versus CVP recording in 90 patients undergoing laparoscopic liver surgery showed a reduced conversion rate as well as reduced blood loss in favor of the SVV approach [232]. The choice for intravenous fluid therapy in liver surgery is still under debate. A systematic review covering 43 RCT compared 18 fluid types (9 crystalloids and 9 colloids) in major abdominal surgery concluded that the best approach was balanced crystalloids (e.g., Ringer’s lactate) as maintenance fluid and colloids as volume expander (e.g., human albumin) [233]. Concerning the postoperative period, a recent retrospective study showed that a weight gain ≥3.5 kg on postoperative day 2 was an independent risk factor for major complication after liver surgery [234]. This suggests that postoperative weight fluctuation should be carefully monitored and potentially minimized.
Summary and recommendation: Low central venous pressure (below 5 cm H2O) with close monitoring is recommended during hepatic transection. As maintenance fluid balanced crystalloid should be preferred over 0.9% saline or colloids. Goal-directed fluid therapy optimizes cardiac output and end-organ perfusion. This may be particularly beneficial after the intraoperative liver resection during a low central venous pressure state to restore tissue perfusion. Patients who have comorbidities and reduced cardiac function may benefit most.
Evidence level: High.
Grade of recommendation: Strong.
Monitoring/audit
Monitoring the outcomes after implementation of ERAS allows performing a precise audit. Outcome monitoring therefore represents the first step to establish an audit of quality. A recent study reported the successful implementation of a nationwide audit for liver surgery in the Netherlands [235]. This audit on postoperative outcomes after liver surgery was intended to evaluate the quality of centers performing these operations and to reach or maintain the best surgical quality. Otherwise, no study specifically designed for liver surgery has been published yet. A Cochrane systematic review on the effects of audit and feedback analyzed 140 studies [236]. It was found that audit and feedback generally induce improvements. The audit was more efficient when the baseline performance was low. The structure of the audit or feedback played a role. It was, for example, of interest to identify specific targets and put in place a plan of action. Another review by Ivers et al. [237] revealed that the body of evidence showing that audit improves outcomes was substantial, but that progress and evolution in this field were not present in recent literature. To a larger scale such as healthcare system, Grimshaw et al. [238] recommended the implementation of laboratories to better understand the science behind audit and feedback and to improve audit and feedback and their impact. One article highlighted the importance of undertaking actions over just measurement [239]. Recently, the Clinical Performance Feedback Intervention Theory issued from a systematic review and meta-synthesis postulated that an effective feedback was a cyclical process and that every missing links stop** the “cycle” cause effect loss of the feedback [240]. This theory includes recommendations for optimally designing or implementing an audit intervention. Practical suggestions on how to effectively display or deliver feedback have also been published [241].
Summary and recommendation: Substantial literature exists supporting that audit and feedback improve outcomes in health care and surgery. Regular audit and feedback should be implemented and performed in liver surgery to monitor and improve postoperative outcomes and compliance to the ERAS program.
Evidence level: Moderate.
Grade of recommendation: Strong.
Discussion
This systematic review and modified Delphi consensus elaborated 25 recommendations based on the best available evidence published until mid-2020. Nine items had a high level of evidence: preoperative smoking and alcohol cessation, preoperative nutrition, wound catheter and TAP block, prophylactic nasogastric intubation, prophylactic abdominal drainage, postoperative artificial nutrition and early oral intake, postoperative glycemic control, PONV prophylaxis, and fluid management.
Regarding differences with 2016 recommendations, more evidence regarding use of steroids before hepatectomy has been published since. It is now routinely recommended in non-diabetic patients. Routine drainage after hepatectomy without biliary reconstruction is not recommended in the present guidelines, whereas in 2016 no conclusive evidence and no recommendation for or against the use of drain were given. In addition, 3 novel items were introduced: prehabilitation, preoperative biliary drainage, and preoperative smoking and alcohol cessation. The novelties of these guidelines are the addition of the 3 novel items mentioned hereabove and the reassessment of the previously published items based on the most recent literature data.
It is not clear if patients with cirrhosis undergoing liver surgery should be managed differently within an ERAS program. Preliminary data showed that ERAS was safe in these patients [242, 243]. Further robust data are needed, and it remains unclear if ERAS pathways should be adapted in cirrhotic patients undergoing liver surgery.
It is important to mention that the compliance (adherence) to all ERAS items is paramount. It has been clearly shown that higher compliance to the ERAS pathway allows to have better postoperative outcomes compared to lower compliance [244, 245].
In conclusion, these guidelines for perioperative care after liver surgery were developed based on the best available evidence and recommend management for 25 perioperative items.
References
Coolsen MME, van Dam RM, van der Wilt AA et al (2013) Systematic review and meta-analysis of enhanced recovery after pancreatic surgery with particular emphasis on pancreaticoduodenectomies. World J Surg 37:1909–1918. https://doi.org/10.1007/s00268-013-2044-3
Greco M, Capretti G, Beretta L et al (2014) Enhanced recovery program in colorectal surgery: a meta-analysis of randomized controlled trials. World J Surg 38:1531–1541. https://doi.org/10.1007/s00268-013-2416-8
Melloul E, Hübner M, Scott M et al (2016) Guidelines for perioperative care for liver surgery: enhanced recovery after surgery (ERAS) society recommendations. World J Surg 40:2425–2440. https://doi.org/10.1007/s00268-016-3700-1
Liang X, Ying H, Wang H et al (2016) Enhanced recovery program versus traditional care in laparoscopic hepatectomy. Medicine (Baltimore) 95:e2835
Liang X, Ying H, Wang H et al (2018) Enhanced recovery care versus traditional care after laparoscopic liver resections: a randomized controlled trial. Surg Endosc 32:2746–2757
Li L, Chen J, Liu Z et al (2017) Enhanced recovery program versus traditional care after hepatectomy: a meta-analysis. Medicine (Baltimore) 96:e8052
Zhao Y, Qin H, Wu Y, **ang B (2017) Enhanced recovery after surgery program reduces length of hospital stay and complications in liver resection: a PRISMA-compliant systematic review and meta-analysis of randomized controlled trials. Medicine (Baltimore) 96:e7628
Noba L, Rodgers S, Chandler C et al (2020) Enhanced recovery after surgery (ERAS) reduces hospital costs and improve clinical outcomes in liver surgery: a systematic review and meta-analysis. J Gastrointest Surg 24:918–932
Brindle M, Nelson G, Lobo DN et al (2020) Recommendations from the ERAS® Society for standards for the development of enhanced recovery after surgery guidelines. BJS Open 4:157–163
Balshem H, Helfand M, Schünemann HJ et al (2011) GRADE guidelines: 3. Rating the quality of evidence. J Clin Epidemiol 64:401–406
Klaiber U, Stephan-Paulsen LM, Bruckner T et al (2018) Impact of preoperative patient education on the prevention of postoperative complications after major visceral surgery: the cluster randomized controlled PEDUCAT trial. Trials 19:288
Pickens R, Cochran A, Tezber K et al (2019) Using a mobile application for real-time collection of patient-reported outcomes in hepatopancreatobiliary surgery within an ERAS® pathway. Am Surg 85:909–917
Hounsome J, Lee A, Greenhalgh J et al (2017) A systematic review of information format and timing before scheduled adult surgery for peri-operative anxiety. Anaesthesia 72:1265–1272
Dagorno C, Sommacale D, Laurent A, et al (2021) Prehabilitation in hepato-pancreato-biliary surgery: a systematic review and meta-analysis. A necessary step forward evidence-based sample size calculation for future trials. J Visc Surg S1878–7886(21)00111–9
Dewulf M, Verrips M, Coolsen MME et al (2021) The effect of prehabilitation on postoperative complications and postoperative hospital stay in hepatopancreatobiliary surgery a systematic review. HPB (Oxford) 23:1299–1310
Doherty DT, Coe PO, Rimmer L et al (2019) Hepatic steatosis in patients undergoing resection of colorectal liver metastases: a target for prehabilitation? A narrative review. Surg Oncol 30:147–158
Bongers BC, Dejong CHC, den Dulk M (2020) Enhanced recovery after surgery programmes in older patients undergoing hepatopancreatobiliary surgery: What benefits might prehabilitation have? Eur J Surg Oncol 47:551–559
Walcott-Sapp S, Billingsley KG (2018) Preoperative optimization for major hepatic resection. Langenbecks Arch Surg 403:23–35
Kaibori M, Ishizaki M, Matsui K et al (2013) Perioperative exercise for chronic liver injury patients with hepatocellular carcinoma undergoing hepatectomy. Am J Surg 206:202–209
Dunne DFJ, Jack S, Jones RP et al (2016) Randomized clinical trial of prehabilitation before planned liver resection. Br J Surg 103:504–512
Nakajima H, Yokoyama Y, Inoue T et al (2019) Clinical benefit of preoperative exercise and nutritional therapy for patients undergoing hepato-pancreato-biliary surgeries for malignancy. Ann Surg Oncol 26:264–272
Wang B, Shelat VG, Chow JJL et al (2020) Prehabilitation program improves outcomes of patients undergoing elective liver resection. J Surg Res 251:119–125
Thillainadesan J, Yumol MF, Hilmer S et al (2020) Interventions to improve clinical outcomes in older adults admitted to a surgical service: a systematic review and meta-analysis. J Am Med Dir Assoc 21:1833–1843
Hijazi Y, Gondal U, Aziz O (2017) A systematic review of prehabilitation programs in abdominal cancer surgery. Int J Surg 39:156–162
Lau CSM, Chamberlain RS (2019) Prehabilitation programs improve exercise capacity before and after surgery in gastrointestinal cancer surgery patients: a meta-analysis. J Gastrointest Surg 24:2829–2837
Kamarajah SK, Bundred J, Weblin J, Tan BHL (2020) Critical appraisal on the impact of preoperative rehabilitation and outcomes after major abdominal and cardiothoracic surgery: a systematic review and meta-analysis. Surgery 167:540–549
Heger P, Probst P, Wiskemann J et al (2020) A systematic review and meta-analysis of physical exercise prehabilitation in major abdominal surgery (PROSPERO 2017 CRD42017080366). J Gastrointest Surg 24:1375–1385
Thomas G, Tahir MR, Bongers BC et al (2019) Prehabilitation before major intra-abdominal cancer surgery: a systematic review of randomised controlled trials. Eur J Anaesthesiol 36:933–945
Hughes MJ, Hackney RJ, Lamb PJ et al (2019) Prehabilitation before major abdominal surgery: a systematic review and meta-analysis. World J Surg 43:1661–1668. https://doi.org/10.1007/s00268-019-04950-y
Luther A, Gabriel J, Watson RP, Francis NK (2018) The impact of total body prehabilitation on post-operative outcomes after major abdominal surgery: a systematic review. World J Surg 42:2781–2791. https://doi.org/10.1007/s00268-018-4569-y
Moran J, Guinan E, McCormick P et al (2016) The ability of prehabilitation to influence postoperative outcome after intra-abdominal operation: a systematic review and meta-analysis. Surgery 160:1189–1201
Celotti A, Solaini L, Montori G et al (2017) Preoperative biliary drainage in hilar cholangiocarcinoma: systematic review and meta-analysis. Eur J Surg Oncol 43:1628–1635
Mehrabi A, Khajeh E, Ghamarnejad O et al (2020) Meta-analysis of the efficacy of preoperative biliary drainage in patients undergoing liver resection for perihilar cholangiocarcinoma. Eur J Radiol 125:108897
Moole H, Bechtold M, Puli SR (2016) Efficacy of preoperative biliary drainage in malignant obstructive jaundice: a meta-analysis and systematic review. World J Surg Oncol 14:182
Aly EA, Johnson CD (2001) Preoperative biliary drainage before resection in obstructive jaundice. Dig Surg 18:84–89
Su CH, Tsay SH, Wu CC et al (1996) Factors influencing postoperative morbidity, mortality, and survival after resection for hilar cholangiocarcinoma. Ann Surg 223:384–394
Mansour JC, Aloia TA, Crane CH et al (2015) Hilar cholangiocarcinoma: expert consensus statement. HPB (Oxford) 17:691–699
Al Mahjoub A, Menahem B, Fohlen A et al (2017) Preoperative biliary drainage in patients with resectable perihilar cholangiocarcinoma: is percutaneous transhepatic biliary drainage safer and more effective than endoscopic biliary drainage? A meta-analysis. J Vasc Interv Radiol 28:576–582
Hameed A, Pang T, Chiou J et al (2016) Percutaneous vs. endoscopic pre-operative biliary drainage in hilar cholangiocarcinoma—a systematic review and meta-analysis. HPB (Oxford) 18:400–410
Tang Z, Yang Y, Meng W, Li X (2017) Best option for preoperative biliary drainage in Klatskin tumor: a systematic review and meta-analysis. Medicine (Baltimore) 96:e8372
Wang L, Lin N, **n F et al (2019) A systematic review of the comparison of the incidence of seeding metastasis between endoscopic biliary drainage and percutaneous transhepatic biliary drainage for resectable malignant biliary obstruction. World J Surg Oncol 17:116
Son JH, Kim J, Lee SH et al (2013) The optimal duration of preoperative biliary drainage for periampullary tumors that cause severe obstructive jaundice. Am J Surg 206:40–46
Grønkjær M, Eliasen M, Skov-Ettrup LS et al (2014) Preoperative smoking status and postoperative complications: a systematic review and meta-analysis. Ann Surg 259:52–71
Lv Y, Liu C, Wei T et al (2015) Cigarette smoking increases risk of early morbidity after hepatic resection in patients with hepatocellular carcinoma. Eur J Surg Oncol 41:513–519
Lindström D, Sadr Azodi O, Wladis A et al (2008) Effects of a perioperative smoking cessation intervention on postoperative complications: a randomized trial. Ann Surg 248:739–745
Møller AM, Villebro N, Pedersen T, Tønnesen H (2002) Effect of preoperative smoking intervention on postoperative complications: a randomised clinical trial. Lancet 359:114–117
Wong J, Lam DP, Abrishami A et al (2012) Short-term preoperative smoking cessation and postoperative complications: a systematic review and meta-analysis. Can J Anaesth 59:268–279
Thomsen T, Villebro N, Møller AM (2014) Interventions for preoperative smoking cessation. Cochrane Database Syst Rev 2014:CD002294
Kai K, Koga H, Aishima S et al (2017) Impact of smoking habit on surgical outcomes in non-B non-C patients with curative resection for hepatocellular carcinoma. World J Gastroenterol 23:1397–1405
Zhang X-F, Wei T, Liu X-M et al (2014) Impact of cigarette smoking on outcome of hepatocellular carcinoma after surgery in patients with hepatitis B. PLOS ONE 9:e85077
Eliasen M, Grønkjær M, Skov-Ettrup LS et al (2013) Preoperative alcohol consumption and postoperative complications: a systematic review and meta-analysis. Ann Surg 258:930–942
Bennett K, Enki DG, Thursz M et al (2019) Systematic review with meta-analysis: high mortality in patients with non-severe alcoholic hepatitis. Aliment Pharmacol Ther 50:249–257
Bo Y, Yao M, Zhang L et al (2015) Preoperative Nutritional Risk Index to predict postoperative survival time in primary liver cancer patients. Asia Pac J Clin Nutr 24:591–597
Fan X, Chen G, Li Y et al (2021) The preoperative prognostic nutritional index in hepatocellular carcinoma after curative hepatectomy: a retrospective cohort study and meta-analysis. J Invest Surg 34:826–833
Man Z, Pang Q, Zhou L et al (2018) Prognostic significance of preoperative prognostic nutritional index in hepatocellular carcinoma: a meta-analysis. HPB (Oxford) 20:888–895
Takagi K, Domagala P, Polak WG et al (2019) Prognostic significance of the controlling nutritional status (CONUT) score in patients undergoing hepatectomy for hepatocellular carcinoma: a systematic review and meta-analysis. BMC Gastroenterol 19:211
Weimann A, Braga M, Carli F et al (2017) ESPEN guideline: clinical nutrition in surgery. Clin Nutr 36:623–650
Masuda T, Shirabe K, Yoshiya S et al (2013) Nutrition support and infections associated with hepatic resection and liver transplantation in patients with chronic liver disease. JPEN J Parenter Enteral Nutr 37:318–326
McKay BP, Larder AL, Lam V (2019) Pre-operative vs. peri-operative nutrition supplementation in hepatic resection for cancer: a systematic review. Nutr Cancer 71:179–198
Barth R, Mills J, Suriawinata A et al (2019) Short-term preoperative diet decreases bleeding after partial hepatectomy: results from a multi-institutional randomized controlled trial. Ann Surg 269:48–52
Mikagi K, Kawahara R, Kinoshita H, Aoyagi S (2011) Effect of preoperative immunonutrition in patients undergoing hepatectomy; a randomized controlled trial. Kurume Med J 58:1–8
Wu Z, Qin J, Pu L (2012) Omega-3 fatty acid improves the clinical outcome of hepatectomized patients with hepatitis B virus (HBV)-associated hepatocellular carcinoma. J Biomed Res 26:395–399
Yang Y, Shao C, Zhang W et al (2019) Omega-3 polyunsaturated fatty acids prevent progression of liver fibrosis and promote liver regeneration after partial hepatectomy in cirrhotic rats. Eur Rev Med Pharmacol Sci 23:10151–10160
Akbari M, Celik SU, Kocaay AF et al (2018) Omega-3 fatty acid supplementation does not influence liver regeneration in rats after partial hepatectomy. Clin Exp Hepatol 4:253–259
Beppu T, Nitta H, Hayashi H et al (2015) Effect of branched-chain amino acid supplementation on functional liver regeneration in patients undergoing portal vein embolization and sequential hepatectomy: a randomized controlled trial. J Gastroenterol 50:1197–1205
Zhang C, Chen B, Jiao A et al (2017) The benefit of immunonutrition in patients undergoing hepatectomy: a systematic review and meta-analysis. Oncotarget 8:86843–86852
Zhang C, Lin J, Li F et al (2019) Effect of omega-3 polyunsaturated fatty acids on liver function and inflammatory reaction in patients undergoing hepatectomy: a systematic review and meta-analysis of randomized control trials. Expert Rev 13:375–384
Meng J, Zhong J, Zhang H et al (2014) Pre-, peri-, and postoperative oral administration of branched-chain amino acids for primary liver cancer patients for hepatic resection: a systematic review. Nutr Cancer 66:517–522
Russell K, Zhang HG, Gillanders LK et al (2019) Preoperative immunonutrition in patients undergoing liver resection: a prospective randomized trial. World J Hepatol 11:305–317
Linecker M, Botea F, Aristotele Raptis D et al (2019) Perioperative omega-3 fatty acids fail to confer protection in liver surgery: results of a multicentric, double-blind, randomized controlled trial. J Hepatol 15:15
Ichikawa K, Okabayashi T, Maeda H et al (2013) Oral supplementation of branched-chain amino acids reduces early recurrence after hepatic resection in patients with hepatocellular carcinoma: a prospective study. Surg Today 43:720–726
American Society of Anesthesiologists (2017) Practice guidelines for preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration: application to healthy patients undergoing elective procedures an updated report by the American Society of Anesthesiologists task force on preoperative fasting and the use of pharmacologic agents to reduce the risk of pulmonary aspiration. Anesthesiology 126:376–393
Scott MJ, Fawcett WJ (2014) Oral carbohydrate preload drink for major surgery—the first steps from famine to feast. Anaesthesia 69:1308–1313
Svanfeldt M, Thorell A, Hausel J et al (2007) Randomized clinical trial of the effect of preoperative oral carbohydrate treatment on postoperative whole-body protein and glucose kinetics. Br J Surg 94:1342–1350
Gjessing PF, Constantin-Teodosiu D, Hagve M et al (2015) Preoperative carbohydrate supplementation attenuates post-surgery insulin resistance via reduced inflammatory inhibition of the insulin-mediated restraint on muscle pyruvate dehydrogenase kinase 4 expression. Clin Nutr 34:1177–1183
Gjessing PF, Hagve M, Fuskevåg O-M et al (2015) Single-dose carbohydrate treatment in the immediate preoperative phase diminishes development of postoperative peripheral insulin resistance. Clin Nutr 34:156–164
Okabayashi T, Nishimori I, Yamashita K et al (2010) Preoperative oral supplementation with carbohydrate and branched-chain amino acid-enriched nutrient improves insulin resistance in patients undergoing a hepatectomy: a randomized clinical trial using an artificial pancreas. Amino Acids 38:901–907
Kobayashi K, Kaneko J, Yamaguchi T et al (2019) Late-evening carbohydrate and branched-chain amino acid snacks improve the nutritional status of patients undergoing hepatectomy based on bioelectrical impedance analysis of body composition. Gastrointest Tumors 6:81–91
Smith MD, McCall J, Plank L, et al (2014) Preoperative carbohydrate treatment for enhancing recovery after elective surgery. Cochrane Database Syst Rev CD009161
Amer MA, Smith MD, Herbison GP et al (2017) Network meta-analysis of the effect of preoperative carbohydrate loading on recovery after elective surgery. Br J Surg 104:187–197
Mathur S, Plank LD, McCall JL et al (2010) Randomized controlled trial of preoperative oral carbohydrate treatment in major abdominal surgery. Br J Surg 97:485–494
Noba L, Wakefield A (2019) Are carbohydrate drinks more effective than preoperative fasting: a systematic review of randomised controlled trials. J Clin Nurs 28:3096–3116
Beyer TA, Werner S (2008) The cytoprotective Nrf2 transcription factor controls insulin receptor signaling in the regenerating liver. Cell Cycle 7:874–878
Gustafsson UO, Nygren J, Thorell A et al (2008) Pre-operative carbohydrate loading may be used in type 2 diabetes patients. Acta Anaesthesiol Scand 52:946–951
Walker KJ, Smith AF (2009) Premedication for anxiety in adult day surgery. Cochrane Database Syst Rev CD002192
By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel (2019) American Geriatrics Society 2019 Updated AGS Beers Criteria® for Potentially Inappropriate Medication Use in Older Adults. J Am Geriatr Soc 67:674–694
Verret M, Lauzier F, Zarychanski R et al (2020) Perioperative use of gabapentinoids for the management of postoperative acute pain: a systematic review and meta-analysis. Anesthesiology 133:265–279
Gan TJ, Belani KG, Bergese S et al (2020) Fourth consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg 131:411–448
Melloul E, Dondéro F, Vilgrain V et al (2012) Pulmonary embolism after elective liver resection: a prospective analysis of risk factors. J Hepatol 57:1268–1275
Eguchi H, Kawamoto K, Tsujie M et al (2020) A prospective, multi-center phase i study of postoperative enoxaparin treatment in patients undergoing curative hepatobiliary-pancreatic surgery for malignancies. Dig Surg 37:81–86
Baltatzis M, Low R, Stathakis P et al (2017) Efficacy and safety of pharmacological venous thromboembolism prophylaxis following liver resection: a systematic review and meta-analysis. HPB (Oxford) 19:289–296
Felder S, Rasmussen MS, King R et al (2019) Prolonged thromboprophylaxis with low molecular weight heparin for abdominal or pelvic surgery. Cochrane Database Syst Rev 8:CD004318
Ho KM, Tan JA (2013) Stratified meta-analysis of intermittent pneumatic compression of the lower limbs to prevent venous thromboembolism in hospitalized patients. Circulation 128:1003–1020
Morris RJ, Woodcock JP (2010) Intermittent pneumatic compression or graduated compression stockings for deep vein thrombosis prophylaxis? A systematic review of direct clinical comparisons. Ann Surg 251:393–396
Gould MK, Garcia DA, Wren SM et al (2012) Prevention of VTE in nonorthopedic surgical patients: antithrombotic therapy and prevention of thrombosis: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines. Chest 141:e227S-e277S
Yang L, Zhang Z, Kong J, Wang W (2019) Systematic review and meta-analysis of the benefit and safety of preoperative administration of steroid in patients undergoing liver resection. Front Pharmacol 10:1442
Hasegawa Y, Nitta H, Takahara T et al (2019) Glucocorticoid use and ischemia-reperfusion injury in laparoscopic liver resection: Randomized controlled trial. Ann Gastroenterol Surg 4:76–83
Bressan AK, Isherwood S, Bathe OF et al (2022) Preoperative single-dose methylprednisolone prevents surgical site infections after major liver resection: a randomized controlled trial. Ann Surg 275:281–287
Brustia R, Fleres F, Tamby E et al (2020) Postoperative collections after liver surgery: Risk factors and impact on long-term outcomes. J Visc Surg 157:199–209
Margonis GA, Sasaki K, Andreatos N et al (2017) Prognostic impact of complications after resection of early stage hepatocellular carcinoma. J Surg Oncol 115:791–804
Matsuda A, Matsumoto S, Seya T et al (2013) Does postoperative complication have a negative impact on long-term outcomes following hepatic resection for colorectal liver metastasis? A meta-analysis. Ann Surg Oncol 20:2485–2492
Moreno Elola-Olaso A, Davenport DL, Hundley JC et al (2012) Predictors of surgical site infection after liver resection: a multicentre analysis using National Surgical Quality Improvement Program data. HPB (Oxford) 14:136–141
Bratzler DW, Houck PM, Workgroup SIPGW (2005) Antimicrobial prophylaxis for surgery: an advisory statement from the National Surgical Infection Prevention Project. Am J Surg 189:395–404
Guo T, Ding R, Yang J et al (2019) Evaluation of different antibiotic prophylaxis strategies for hepatectomy: a network meta-analysis. Medicine (Baltimore) 98:e16241
Zhou YM, Chen ZY, Li XD et al (2015) Preoperative antibiotic prophylaxis does not reduce the risk of postoperative infectious complications in patients undergoing elective hepatectomy. Dig Dis Sci 61:1707–13
Hirokawa F, Hayashi M, Miyamoto Y et al (2013) Evaluation of postoperative antibiotic prophylaxis after liver resection: a randomized controlled trial. Am J Surg 206:8–15
Takayama T, Aramaki O, Shibata T et al (2019) Antimicrobial prophylaxis for 1 day versus 3 days in liver cancer surgery: a randomized controlled non-inferiority trial. Surg Today 49:859–869
Okamura K, Tanaka K, Miura T et al (2017) Randomized controlled trial of perioperative antimicrobial therapy based on the results of preoperative bile cultures in patients undergoing biliary reconstruction. J Hepatobiliary Pancreat Sci 24:382–393
Sugawara G, Yokoyama Y, Ebata T et al (2018) Duration of antimicrobial prophylaxis in patients undergoing major hepatectomy with extrahepatic bile duct resection: a randomized controlled trial. Ann Surg 267:142–148
Ramanathan R, Borrebach J, Tohme S, Tsung A (2018) Preoperative biliary drainage is associated with increased complications after liver resection for proximal cholangiocarcinoma. J Gastrointest Surg 22:1950–1957
Takahashi Y, Takesue Y, Fujiwara M et al (2018) Risk factors for surgical site infection after major hepatobiliary and pancreatic surgery. J Infect Chemother 24:739–743
Hsieh CS, Cheng HC, Lin JS et al (2014) Effect of 4% chlorhexidine gluconate predisinfection skin scrub prior to hepatectomy: a double-blinded, randomized control study. Int Surg 99:787–794
Darouiche RO, Wall MJ, Itani KMF et al (2010) Chlorhexidine-alcohol versus povidone-iodine for surgical-site antisepsis. N Engl J Med 362:18–26
Buell JF, Cherqui D, Geller DA et al (2009) The international position on laparoscopic liver surgery: the Louisville statement, 2008. Ann Surg 250:825–830
Wakabayashi G, Cherqui D, Geller DA et al (2015) Recommendations for laparoscopic liver resection: a report from the second international consensus conference held in Morioka. Ann Surg 261:619–629
Hilal MA, Aldrighetti L, Dagher I et al (2018) The Southampton consensus guidelines for laparoscopic liver surgery: from indication to implementation. Ann Surg 268:11–18
Fretland AA, Dagenborg VJ, Bjornelv GMW et al (2018) Laparoscopic versus open resection for colorectal liver metastases: the OSLO-COMET randomized controlled trial. Ann Surg 267:199–207
Robles-Campos R, Lopez-Lopez V, Brusadin R et al (2019) Open versus minimally invasive liver surgery for colorectal liver metastases (LapOpHuva): a prospective randomized controlled trial. Surg Endosc 33:3926–3936
Ciria R, Cherqui D, Geller DA et al (2016) Comparative short-term benefits of laparoscopic liver resection: 9000 cases and climbing. Ann Surg 263:761–777
Ciria R, Gomez-Luque I, Ocana S et al (2019) A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for hepatocellular carcinoma: updated results from the European Guidelines meeting on Laparoscopic Liver Surgery, Southampton, UK, 2017. Ann Surg Oncol 26:252–263
Ciria R, Ocana S, Gomez-Luque I et al (2020) A systematic review and meta-analysis comparing the short- and long-term outcomes for laparoscopic and open liver resections for liver metastases from colorectal cancer. Surg Endosc 34:349–360
Syn NL, Kabir T, Koh YX et al (2019) Survival advantage of laparoscopic versus open resection for colorectal liver metastases: a meta-analysis of individual patient data from randomized trials and propensity-score matched studies. Ann Surg 22:22
Okumura S, Goumard C, Gayet B et al (2019) Laparoscopic versus open two-stage hepatectomy for bilobar colorectal liver metastases: a bi-institutional, propensity score-matched study. Surgery 166:959–966
Cipriani F, Alzoubi M, Fuks D et al (2020) Pure laparoscopic versus open hemihepatectomy: a critical assessment and realistic expectations—a propensity score-based analysis of right and left hemihepatectomies from nine European tertiary referral centers. J Hepatobiliary Pancreat Sci 27:3–15
Soubrane O, de Rougemont O, Kim K-H et al (2015) Laparoscopic living donor left lateral sectionectomy: a new standard practice for donor hepatectomy. Ann Surg 262:757–761
Soubrane O, Eguchi S, Uemoto S et al (2022) Minimally invasive donor hepatectomy for adult living donor liver transplantation: an international, multi-institutional evaluation of safety, efficacy and early outcomes. Ann Surg 275:166–174
Scatton O, Katsanos G, Boillot O et al (2015) Pure laparoscopic left lateral sectionectomy in living donors: from innovation to development in France. Ann Surg 261:506–512
Vicente D, Patino M, Marcus R et al (2019) Impact of epidural analgesia on the systemic biomarker response after hepatic resection. Oncotarget 10:584–594
Kambakamba P, Slankamenac K, Tschuor C et al (2015) Epidural analgesia and perioperative kidney function after major liver resection. Br J Surg 102:805–812
Sakowska M, Docherty E, Linscott D, Connor S (2009) A change in practice from epidural to intrathecal morphine analgesia for hepato-pancreato-biliary surgery. World J Surg 33:1802–1808. https://doi.org/10.1007/s00268-009-0131-2
Aloia TA, Kim BJ, Segraves-Chun YS et al (2017) A randomized controlled trial of postoperative thoracic epidural analgesia versus intravenous patient-controlled analgesia after major hepatopancreatobiliary surgery. Ann Surg 266:545–554
Salicath JH, Yeoh EC, Bennett MH (2018) Epidural analgesia versus patient-controlled intravenous analgesia for pain following intra-abdominal surgery in adults. Cochrane Database Syst Rev 8:CD010434
Niewinski G, Figiel W, Grat M et al (2020) A comparison of intrathecal and intravenous morphine for analgesia after hepatectomy: a randomized controlled trial. World J Surg 44:2340–2349. https://doi.org/10.1007/s00268-020-05437-x
Tang JZJ, Weinberg L (2019) A literature review of intrathecal morphine analgesia in patients undergoing major open hepato-pancreatic-biliary (Hpb) surgery. Anesthesiol Pain Med 9:e94441
Chen MT, ** B, Du SD et al (2017) Role of a selective cyclooxygenase-2 inhibitor on pain and enhanced recovery after open hepatectomy: a randomized controlled trial. Transl Cancer Res 6:806–814
Yassen AM, Sayed GE (2012) Low dose ketorolac infusion improves postoperative analgesia combined with patient controlled fentanyl analgesia after living donor hepatectomy—randomized controlled trial. Egypt J Anaesth 28:199–204
Hughes MJ, Harrison EM, ** Y et al (2015) Acetaminophen metabolism after liver resection: a prospective case-control study. Dig Liver Dis 47:1039–1046
Liu Y, Song X, Sun D et al (2018) Evaluation of intravenous parecoxib infusion pump of patient-controlled analgesia compared to fentanyl for postoperative pain management in laparoscopic liver resection. Med Sci Monit 24:8224–8231
Revie EJ, McKeown DW, Wilson JA et al (2012) Randomized clinical trial of local infiltration plus patient-controlled opiate analgesia vs. epidural analgesia following liver resection surgery. HPB (Oxford) 14:611–618
Bell R, Ward D, Jeffery J et al (2019) A randomized controlled trial comparing epidural analgesia versus continuous local anesthetic infiltration via abdominal wound catheter in open liver resection. Ann Surg 269:413–419
Hughes MJ, Harrison EM, Peel NJ et al (2015) Randomized clinical trial of perioperative nerve block and continuous local anaesthetic infiltration via wound catheter versus epidural analgesia in open liver resection (LIVER 2 trial). Br J Surg 102:1619–1628
Mungroop TH, Veelo DP, Busch OR et al (2016) Continuous wound infiltration versus epidural analgesia after hepato-pancreato-biliary surgery (POP-UP): a randomised controlled, open-label, non-inferiority trial. Lancet Gastroenterol Hepatol 1:105–113
Karanicolas PJ, Cleary S, McHardy P et al (2018) Medial open transversus abdominis plane (MOTAP) catheters reduce opioid requirements and improve pain control following open liver resection: a multicenter, blinded, randomized controlled trial. Ann Surg 268:233–240
Chan SK, Lai PB, Li PT et al (2010) The analgesic efficacy of continuous wound instillation with ropivacaine after open hepatic surgery. Anaesthesia 65:1180–1186
Sun JX, Bai KY, Liu YF et al (2017) Effect of local wound infiltration with ropivacaine on postoperative pain relief and stress response reduction after open hepatectomy. World J Gastroenterol 23:6733–6740
Lee SH, Gwak MS, Choi SJ et al (2013) Prospective, randomized study of ropivacaine wound infusion versus intrathecal morphine with intravenous fentanyl for analgesia in living donors for liver transplantation. Liver Transpl 19:1036–1045
Peres-Bachelot V, Blanc E, Oussaid N et al (2019) A 96-hour continuous wound infiltration with ropivacaine reduces analgesic consumption after liver resection: a randomized, double-blind, controlled trial. J Surg Oncol 119:47–55
Yu H, Li ZY, Yu X (2013) Efficacy of postoperative continuous wound infiltration with local anesthesia after open hepatectomy. Zhonghua Yi Xue Za Zhi 93:2723–2726
**n Y, Hong Y, Yong LZ (2014) Efficacy of postoperative continuous wound infiltration with local anesthesia after open hepatectomy. Clin J Pain 30:571–576
Dalmau A, Fustran N, Camprubi I et al (2018) Analgesia with continuous wound infusion of local anesthetic versus saline: double-blind randomized, controlled trial in hepatectomy. Am J Surg 215:138–143
Niraj G, Kelkar A, Jeyapalan I et al (2011) Comparison of analgesic efficacy of subcostal transversus abdominis plane blocks with epidural analgesia following upper abdominal surgery. Anaesthesia 66:465–471
Shaker TM, Carroll JT, Chung MH et al (2018) Efficacy and safety of transversus abdominis plane blocks versus thoracic epidural anesthesia in patients undergoing major abdominal oncologic resections: a prospective, randomized controlled trial. Am J Surg 215:498–501
Abdelsalam K, Mohamdin OW (2016) Ultrasound-guided rectus sheath and transversus abdominis plane blocks for perioperative analgesia in upper abdominal surgery: a randomized controlled study. Saudi J Anaesth 10:25–28
Erdogan MA, Ozgul U, Ucar M et al (2017) Effect of transversus abdominis plane block in combination with general anesthesia on perioperative opioid consumption, hemodynamics, and recovery in living liver donors: the prospective, double-blinded, randomized study. Clin Transpl 31:e12931
Guo JG, Li HL, Pei QQ, Feng ZY (2018) The analgesic efficacy of subcostal transversus abdominis plane block with Mercedes incision. BMC Anesthesiol 18:36
Serag Eldin M, Mahmoud F, El Hassan R et al (2014) Intravenous patient-controlled fentanyl with and without transversus abdominis plane block in cirrhotic patients post liver resection. Local Reg 7:27–37
Yassen K, Lotfy M, Miligi A et al (2019) Patient-controlled analgesia with and without transverse abdominis plane and rectus sheath space block in cirrhotic patients undergoing liver resection. J Anesthesiol Clin Pharmacol 35:58–64
Zhu Q, Li L, Yang Z et al (2019) Ultrasound guided continuous Quadratus Lumborum block hastened recovery in patients undergoing open liver resection: a randomized controlled, open-label trial. BMC Anesthesiol 19:23
Zhang H, Du G, Liu YF et al (2019) Overlay of a sponge soaked with ropivacaine and multisite infiltration analgesia result in faster recovery after laparoscopic hepatectomy. World J Gastroenterol 25:5185–5196
Li H, Chen R, Yang Z et al (2018) Comparison of the postoperative effect between epidural anesthesia and continuous wound infiltration on patients with open surgeries: a meta-analysis. J Clin Anesth 51:20–31
Gavriilidis P, Roberts KJ, Sutcliffe RP (2019) Local anaesthetic infiltration via wound catheter versus epidural analgesia in open hepatectomy: a systematic review and meta-analysis of randomised controlled trials. HPB (Oxford) 21:945–952
Ichida H, Imamura H, Yoshimoto J et al (2016) Randomized controlled trial for evaluation of the routine use of nasogastric tube decompression after elective liver surgery. J Gastrointest Surg 20:1324–1330
Wen Z, Zhang X, Liu Y et al (2019) Is routine nasogastric decompression after hepatic surgery necessary? A systematic review and meta-analysis. Int J Nurs Stud 100:103406
Kim YI, Fujita S, Hwang VJ, Nagase Y (2014) Comparison of abdominal drainage and no-drainage after elective hepatectomy: a randomized study. Hepatogastroenterology 61:707–711
Butte JM, Grendar J, Bathe O et al (2014) The role of peri-hepatic drain placement in liver surgery: a prospective analysis. HPB (Oxford) 16:936–942
Messager M, Sabbagh C, Denost Q et al (2015) Is there still a need for prophylactic intra-abdominal drainage in elective major gastro-intestinal surgery? J Visc Surg 152:305–313
Gavriilidis P, Hidalgo E, de’Angelis N et al (2017) Re-appraisal of prophylactic drainage in uncomplicated liver resections: a systematic review and meta-analysis. HPB (Oxford) 19:16–20
Petrowsky H, Demartines N, Rousson V, Clavien P-A (2004) Evidence-based value of prophylactic drainage in gastrointestinal surgery: a systematic review and meta-analyses. Ann Surg 240:1074–1084
Zimmitti G, Vauthey J-N, Shindoh J et al (2013) Systematic use of an intraoperative air leak test at the time of major liver resection reduces the rate of postoperative biliary complications. J Am Coll Surg 217:1028–1037
Tran Cao HS, Phuoc V, Ismael H et al (2017) Rate of organ space infection is reduced with the use of an air leak test during major hepatectomies. J Gastrointest Surg 21:85–93
Galvão CM, Liang Y, Clark AM (2010) Effectiveness of cutaneous warming systems on temperature control: meta-analysis. J Adv Nurs 66:1196–1206
Warttig S, Alderson P, Lewis SR, Smith AF (2016) Intravenous nutrients for preventing inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev 11:CD009906
Egan C, Bernstein E, Reddy D et al (2011) A randomized comparison of intraoperative PerfecTemp and forced-air warming during open abdominal surgery. Anesth Analg 113:1076–1081
Campbell G, Alderson P, Smith AF, Warttig S (2015) Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev CD009891
Alderson P, Campbell G, Smith AF, et al (2014) Thermal insulation for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev CD009908
Madrid E, Urrútia G, Roqué i Figuls M et al (2016) Active body surface warming systems for preventing complications caused by inadvertent perioperative hypothermia in adults. Cochrane Database Syst Rev 4:CD009016
Moola S, Lockwood C (2010) The effectiveness of strategies for the management and/or prevention of hypothermia within the adult perioperative environment: systematic review. JBI Libr Syst Rev 8:752–792
Tandon M, Karna ST, Pandey CK et al (2018) Multimodal temperature management during donor hepatectomy under combined general anaesthesia and neuraxial analgesia: retrospective analysis. Indian J Anaesth 62:431–435
Birch DW, Dang JT, Switzer NJ et al (2016) Heated insufflation with or without humidification for laparoscopic abdominal surgery. Cochrane Database Syst Rev 10:CD007821
Olthof PB, Reiniers MJ, Dirkes MC et al (2016) Protective mechanisms of hypothermia in liver surgery and transplantation. Mol Med 21:833–846
Lassen K, Kjaeve J, Fetveit T et al (2008) Allowing normal food at will after major upper gastrointestinal surgery does not increase morbidity: a randomized multicenter trial. Ann Surg 247:721–729
Ciuni R, Biondi A, Grosso G et al (2011) Nutritional aspects in patient undergoing liver resection. Updates Surg 63:249–252
Cornide-Petronio ME, Alvarez-Mercado AI, Jimenez-Castro MB, Peralta C (2020) Current knowledge about the effect of nutritional status, supplemented nutrition diet, and gut microbiota on hepatic ischemia-reperfusion and regeneration in liver surgery. Nutrients 12:284
Hotta T, Kobayashi Y, Taniguchi K et al (2003) Evaluation of postoperative nutritional state after hepatectomy for hepatocellular carcinoma. Hepatogastroenterology 50:1511–1516
Ishikawa Y, Yoshida H, Mamada Y et al (2010) Prospective randomized controlled study of short-term perioperative oral nutrition with branched chain amino acids in patients undergoing liver surgery. Hepatogastroenterology 57:583–590
Chiarla C, Giovannini I, Giuliante F et al (2012) Parenteral nutrition in liver resection. J Nutr Metab 2012:508103
Gao LB, Tian H, Wang XG et al (2015) Early enteral and parenteral nutritional support after hepatectomy in patients with hepatic carcinoma: a systematic review and meta-analysis. Onco Targets Ther 8:623–631
Zhao XF, Wu N, Zhao GQ et al (2016) Enteral nutrition versus parenteral nutrition after major abdominal surgery in patients with gastrointestinal cancer: a systematic review and meta-analysis. J Investig Med 64:1061–1074
Sun Y, Yang Z, Tan H (2014) Perioperative nutritional support and fluid therapy in patients with liver diseases. Hepatobiliary Surg 3:140–148
Frisch A, Chandra P, Smiley D et al (2010) Prevalence and clinical outcome of hyperglycemia in the perioperative period in noncardiac surgery. Diabetes Care 33:1783–1788
King JT, Goulet JL, Perkal MF, Rosenthal RA (2011) Glycemic control and infections in patients with diabetes undergoing noncardiac surgery. Ann Surg 253:158–165
Lipshutz AKM, Gropper MA (2009) Perioperative glycemic control: an evidence-based review. Anesthesiology 110:408–421
Eshuis WJ, Hermanides J, van Dalen JW et al (2011) Early postoperative hyperglycemia is associated with postoperative complications after pancreatoduodenectomy. Ann Surg 253:739–744
Jackson RS, Amdur RL, White JC, Macsata RA (2012) Hyperglycemia is associated with increased risk of morbidity and mortality after colectomy for cancer. J Am Coll Surg 214:68–80
Blixt C, Ahlstedt C, Ljungqvist O et al (2012) The effect of perioperative glucose control on postoperative insulin resistance. Clin Nutr 31:676–681
Maeda H, Okabayashi T, Nishimori I et al (2010) Hyperglycemia during hepatic resection: continuous monitoring of blood glucose concentration. Am J Surg 199:8–13
Okabayashi T, Nishimori I, Maeda H et al (2009) Effect of intensive insulin therapy using a closed-loop glycemic control system in hepatic resection patients: a prospective randomized clinical trial. Diabetes Care 32:1425–1427
Vibert E, Boleslawski E, Cosse C et al (2015) Arterial lactate concentration at the end of an elective hepatectomy is an early predictor of the postoperative course and a potential surrogate of intraoperative events. Ann Surg 262:787–792
Fisette A, Hassanain M, Metrakos P et al (2012) High-dose insulin therapy reduces postoperative liver dysfunction and complications in liver resection patients through reduced apoptosis and altered inflammation. J Clin Endocrinol Metab 97:217–226
Okabayashi T, Shima Y, Sumiyoshi T et al (2014) Intensive versus intermediate glucose control in surgical intensive care unit patients. Diabetes Care 37:1516–1524
Hassanain M, Metrakos P, Fisette A et al (2013) Randomized clinical trial of the impact of insulin therapy on liver function in patients undergoing major liver resection. Br J Surg 100:610–618
Sato H, Lattermann R, Carvalho G et al (2010) Perioperative glucose and insulin administration while maintaining normoglycemia (GIN therapy) in patients undergoing major liver resection. Anesth Analg 110:1711–1718
Takesue Y, Tsuchida T (2017) Strict glycemic control to prevent surgical site infections in gastroenterological surgery. Ann Gastroenterol Surg 1:52–59
Polderman JAW, van Steen SCJ, Thiel B et al (2018) Peri-operative management of patients with type-2 diabetes mellitus undergoing non-cardiac surgery using liraglutide, glucose–insulin–potassium infusion or intravenous insulin bolus regimens: a randomised controlled trial. Anaesthesia 73:332–339
Igami T, Nishio H, Ebata T et al (2011) Using the greater omental flap to cover the cut surface of the liver for prevention of delayed gastric emptying after left-sided hepatobiliary resection: a prospective randomized controlled trial. J Hepatobiliary Pancreat Sci 18:176–183
Yoshida H, Mamada Y, Taniai N et al (2005) Fixation of the greater omentum for prevention of delayed gastric emptying after left-sided hepatectomy: a randomized controlled trial. Hepatogastroenterology 52:1334–1337
Oida T, Mimatsu K, Kawasaki A et al (2010) Fixation of the round ligament to the peritoneum and wrap** of the cut surface of the liver for prevention of early delayed gastric emptying after hepatic lateral segmentectomy. Langenbecks Arch Surg 395:655–659
Shimada M, Morine Y, Nagano H et al (2015) Effect of TU-100, a traditional Japanese medicine, administered after hepatic resection in patients with liver cancer: a multi-center, phase III trial (JFMC40-1001). Int J Clin Oncol 20:95–104
Nishi M, Shimada M, Uchiyama H et al (2012) The beneficial effects of Kampo medicine Dai-ken-chu-to after hepatic resection: a prospective randomized control study. Hepatogastroenterology 59:2290–2294
You XM, Mo XS, Ma L et al (2015) Randomized clinical trial comparing efficacy of simo decoction and acupuncture or chewing gum alone on postoperative ileus in patients with hepatocellular carcinoma after hepatectomy. Medicine (Baltimore) 94:e1968
Hendry PO, van Dam RM, Bukkems SF et al (2010) Randomized clinical trial of laxatives and oral nutritional supplements within an enhanced recovery after surgery protocol following liver resection. Br J Surg 97:1198–1206
Jang SY, Ju EY, Kim DE et al (2012) First flatus time and xerostomia associated with gum-chewing after liver resection. J Clin Nurs 21:2188–2192
Kehlet H, Wilmore DW (2002) Multimodal strategies to improve surgical outcome. Am J Surg 183:630–641
Convertino VA (1997) Cardiovascular consequences of bed rest: effect on maximal oxygen uptake. Med Sci Sports Exerc 29:191–196
Brower RG (2009) Consequences of bed rest. Crit Care Med 37:S422–S428
Ni CY, Wang ZH, Huang ZP et al (2018) Early enforced mobilization after liver resection: a prospective randomized controlled trial. Int J Surg 54:254–258
De Almeida EPM, De Almeida JP, Landoni G et al (2017) Early mobilization programme improves functional capacity after major abdominal cancer surgery: a randomized controlled trial. Br J Anaesth 119:900–907
Burgess LC, Immins T, Wainwright TW (2019) What is the role of post-operative physiotherapy in general surgical Enhanced Recovery after Surgery pathways? Eur J Physiother 21:67–72
Jones C, Kelliher L, Dickinson M et al (2013) Randomized clinical trial on enhanced recovery versus standard care following open liver resection. Br J Surg 100:1015–1024
Apfel CC, Heidrich FM, Jukar-Rao S et al (2012) Evidence-based analysis of risk factors for postoperative nausea and vomiting. Br J Anaesth 109:742–753
Carlisle JB, Stevenson CA (2006) Drugs for preventing postoperative nausea and vomiting. Cochrane Database Syst Rev CD004125
DREAMS Trial Collaborators and West Midlands Research Collaborative (2017) Dexamethasone versus standard treatment for postoperative nausea and vomiting in gastrointestinal surgery: randomised controlled trial (DREAMS Trial). BMJ 357:j1455
Kooby DA, Stockman J, Ben-Porat L et al (2003) Influence of transfusions on perioperative and long-term outcome in patients following hepatic resection for colorectal metastases. Ann Surg 237:860–869
Melendez JA, Arslan V, Fischer ME et al (1998) Perioperative outcomes of major hepatic resections under low central venous pressure anesthesia: blood loss, blood transfusion, and the risk of postoperative renal dysfunction. J Am Coll Surg 187:620–625
Page AJ, Kooby DA (2012) Perioperative management of hepatic resection. J Gastrointest Oncol 3:19–27
Gurusamy KS, Li J, Vaughan J, et al (2012) Cardiopulmonary interventions to decrease blood loss and blood transfusion requirements for liver resection. Cochrane Database Syst Rev CD007338
Hughes MJ, Ventham NT, Harrison EM, Wigmore SJ (2015) Central venous pressure and liver resection: a systematic review and meta-analysis. HPB (Oxford) 17:863–871
Cecconi M, Corredor C, Arulkumaran N et al (2013) Clinical review: Goal-directed therapy-what is the evidence in surgical patients? The effect on different risk groups. Crit Care 17:209
Correa-Gallego C, Tan KS, Arslan-Carlon V et al (2015) Goal-directed fluid therapy using stroke volume variation for resuscitation after low central venous pressure-assisted liver resection: a randomized clinical trial. J Am Coll Surg 221:591–601
Weinberg L, Ianno D, Churilov L et al (2019) Goal directed fluid therapy for major liver resection: a multicentre randomized controlled trial. Ann Med Surg (Lond) 45:45–53
Dunki-Jacobs EM, Philips P, Scoggins CR et al (2014) Stroke volume variation in hepatic resection: a replacement for standard central venous pressure monitoring. Ann Surg Oncol 21:473–478
Ratti F, Cipriani F, Reineke R et al (2016) Intraoperative monitoring of stroke volume variation versus central venous pressure in laparoscopic liver surgery: a randomized prospective comparative trial. HPB (Oxford) 18:136–144
Noonpradej S, Akaraborworn O (2020) Intravenous fluid of choice in major abdominal surgery: a systematic review. Crit Care Res Pract 2020:2170828
Labgaa I, Joliat G-R, Grass F et al (2020) Impact of postoperative weight gain on complications after liver surgery. HPB (Oxford) 22:744–749
van der Werf LR, Kok NFM, Buis CI et al (2019) Implementation and first results of a mandatory, nationwide audit on liver surgery. HPB (Oxford) 21:1400–1410
Ivers N, Jamtvedt G, Flottorp S, et al (2012) Audit and feedback: effects on professional practice and healthcare outcomes. Cochrane Database Syst Rev CD000259
Ivers NM, Grimshaw JM, Jamtvedt G et al (2014) Growing literature, stagnant science? Systematic review, meta-regression and cumulative analysis of audit and feedback interventions in health care. J Gen Intern Med 29:1534–1541
Grimshaw JM, Ivers N, Linklater S et al (2019) Reinvigorating stagnant science: implementation laboratories and a meta-laboratory to efficiently advance the science of audit and feedback. BMJ Qual Saf 28:416–423
Foy R, Skrypak M, Alderson S et al (2020) Revitalising audit and feedback to improve patient care. BMJ 368:m213
Brown B, Gude WT, Blakeman T et al (2019) Clinical performance feedback intervention theory (CP-FIT): a new theory for designing, implementing, and evaluating feedback in health care based on a systematic review and meta-synthesis of qualitative research. Implement Sci 14:40
Brehaut JC, Colquhoun HL, Eva KW et al (2016) Practice feedback interventions: 15 suggestions for optimizing effectiveness. Ann Intern Med 164:435–441
Lunel T, Mohkam K, Merle P et al (2021) Impact of 2016 enhanced recovery after surgery (ERAS) recommendations on outcomes after hepatectomy in cirrhotic and non-cirrhotic patients. World J Surg 45:2964–2974. https://doi.org/10.1007/s00268-021-06229-7
Zheng Y, Wang L, Wu F et al (2020) Enhanced recovery after surgery strategy for cirrhosis patients undergoing hepatectomy: experience in a single research center. Ann Surg Treat Res 98:224–234
Olson KA, Fleming RYD, Fox AW et al (2021) The enhanced recovery after surgery (ERAS) elements that most greatly impact length of stay and readmission. Am Surg 87:473–479
St-Amour P, St-Amour P, Joliat GR et al (2020) Impact of ERAS compliance on the delay between surgery and adjuvant chemotherapy in hepatobiliary and pancreatic malignancies. Langenbecks Arch Surg 405:959–966
Acknowledgements
The authors would like to thank Cécile Jaques and Alexia Trombert, Biomedical Information Specialists (Medical Library, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland), for support with the systematic literature search.
Funding
Open access funding provided by University of Lausanne.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Prof. Nicolas Demartines is implementation chair of the ERAS Society. The other authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Joliat, GR., Kobayashi, K., Hasegawa, K. et al. Guidelines for Perioperative Care for Liver Surgery: Enhanced Recovery After Surgery (ERAS) Society Recommendations 2022. World J Surg 47, 11–34 (2023). https://doi.org/10.1007/s00268-022-06732-5
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00268-022-06732-5