Abstract
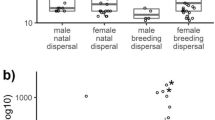
Dispersal of individuals before their first breeding attempt or between subsequent attempts facilitates spatial and temporal gene flow within and among populations. However, in species on oceanic islands, dispersal is often restricted to a single island, and thus, the risk of inbreeding is particularly high in those small, closed, and isolated populations. One of the mechanisms that may prevent inbreeding within island populations is sex-biased dispersal, which results in close kin of the opposite sex not being in the same area for breeding. In this study, we investigated dispersal patterns, and their costs and benefits, in the Chatham Island black robin, a small passerine confined to two small islands. We found that black robins practice a resource defense mating system as male black robins were more likely to divorce than change territory between breeding seasons. Natal dispersal was female-biased in both the proportion of birds dispersing and the distance dispersed. Bird density in the natal year increased the proportion of birds dispersing in both sexes. Breeding success was reduced for females after natal dispersal. Breeding dispersal was rare and female-biased in proportion only. Regardless of sex, black robins were more likely to disperse after losing a mate, but females dispersed further than males. This study suggests that in closed populations of island species with limited habitat, sex-biased density-dependent dispersal may be a mechanism that minimizes inbreeding.
Significance statement
Dispersal of individuals facilitates gene flow within and among populations. However, in species on oceanic islands, dispersal is often restricted to movement within a single island. Few studies have investigated dispersal and its costs and benefits in spatially restricted species. Here, we studied the endangered Chatham Island black robin, a songbird confined to two small islands. We found that on an island, where habitat is extremely limited, males are highly territorial and they are more likely to change partners than territories. Bird density in the natal year forces both young males and females to move, but females are more likely to disperse and move further than males. Sex-biased density-dependent dispersal may be a mechanism that minimizes inbreeding. This is particularly important for threatened island endemic species where maintaining high genetic diversity ensures the population’s long-term viability.




Similar content being viewed by others
References
Aars J, Ims RA (2000) Population dynamic and genetic consequences of spatial density-dependent dispersal in patchy populations. Am Nat 155:252–265
Adams ES (2001) Approaches to the study of territory size and shape. Annu Rev Ecol Syst 32:277–303
Allee WC, Bowen ES (1932) Studies in animal aggregations: mass protection against colloidal silver among goldfishes. J Exp Zool 61:185–207
Arcese P (1989) Intrasexual competition, mating system and natal dispersal in song sparrows. Anim Behav 38:958–979
Ardern SL, Lambert DM (1997) Is the black robin in genetic peril? Mol Ecol 6:21–28
Arlt D, Pärt T (2008) Sex-biased dispersal: a result of a sex difference in breeding site availability. Am Nat 171:844–850
Bates D, Maechler M, Bolker B, Walker S (2016) lme4: linear mixed-effects models using Eigen and S4. R package version 1.1–12 edn, https://cran.r-project.org/web/packages/lme4/index.html
Butler D, Merton D (1992) The black robin: saving the world’s most endangered bird. Oxford University Press, Auckland
Charlesworth B, Morgan MT, Charlesworth D (1993) The effect of deleterious mutations on neutral molecular variation. Genetics 134:1289–1303
Clarke AL, Sæther B-E, Røskaft E (1997) Sex biases in avian dispersal: a reappraisal. Oikos 79:429–438
Clobert J (2001) Dispersal. Oxford University Press, New York
Clobert J, Ims RA, Rousset F (2004) Causes, mechanisms and consequences of dispersal. In: Hanski I, Gaggiotti OE (eds) Ecology, genetics and evolution of metapopulations. Elsevier Science, Burlington, pp. 307–335
Clobert J, Le Galliard J-F, Cote J, Meylan S, Massot M (2009) Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol Lett 12:197–209
Daniels SJ, Walters JR (2000) Inbreeding depression and its effects on natal dispersal in red-cockaded woodpeckers. Condor 102:482–491
Dobson FS, Jones WT (1985) Multiple causes of dispersal. Am Nat 126:855–858
Eikenaar C, Komdeur J, Richardson DS (2008) Natal dispersal patterns are not associated with inbreeding avoidance in the Seychelles warbler. J Evol Biol 21:1106–1116
ESRI (2013) ArcGIS, 10.2 edn. Environmental Systems Resource Institute, Redlands, California
Fortin M-J, Dale M (2005) Spatial analysis: a guide for ecologists. Cambridge University Press, Cambridge
Frankham R, Lees K, Montgomery ME, England PR, Lowe EH, Briscoe DA (1999) Do population size bottlenecks reduce evolutionary potential? Anim Conserv 2:255–260
Goudet J, Perrin N, Waser P (2002) Tests for sex-biased dispersal using bi-parentally inherited genetic markers. Mol Ecol 11:1103–1114
Greenwood PJ (1980) Mating systems, philopatry and dispersal in birds and mammals. Anim Behav 28:1140–1162
Greenwood PJ, Harvey PH (1982) The natal and breeding dispersal of birds. Annu Rev Ecol Syst 13:1–21
Hansson B, Jack L, Christians JK, Pemberton JM, Åkesson M, Westerdahl H, Bensch S, Hasselquist D (2007) No evidence for inbreeding avoidance in a great reed warbler population. Behav Ecol 18:157–164
Hauber ME, Sherman PW (2001) Self-referent phenotype matching: theoretical considerations and empirical evidence. Trends Neurosci 24:609–616
Higgins PJ, Peters JM (2002) Handbook of Australian, New Zealand and Antarctic birds. Pardalotes to shrike-thrushes, vol 6. Oxford University Press, Melbourne
Jamieson IG, Taylor SS, Tracy LN, Kokko H, Armstrong DP (2009) Why some species of birds do not avoid inbreeding: insights from New Zealand robins and saddlebacks. Behav Ecol 20:575–584
Keller LF, Arcese P (1998) No evidence for inbreeding avoidance in a natural population of song sparrows (Melospiza melodia). Am Nat 152:380–392
Keller LF, Waller DM (2002) Inbreeding effects in wild populations. Trends Ecol Evol 17:230–241
Kennedy ES, Grueber CE, Duncan RP, Jamieson IG (2014) Severe inbreeding depression and no evidence of purging in an extremely inbred wild species—the Chatham Island black robin. Evolution 68:987–995
Koenig WD, Van Vuren D, Hooge PN (1996) Detectability, philopatry, and the distribution of dispersal distances in vertebrates. Trends Ecol Evol 11:514–517
Komdeur J (1996) Facultative sex ratio bias in the offspring of Seychelles warblers. Proc R Soc Lond B 263:661–666
Komdeur J, Hatchwell BJ (1999) Kin recognition: function and mechanism in avian societies. Trends Ecol Evol 14:237–241
Lebigre C, Alatalo RV, Siitari H (2010) Female-biased dispersal alone can reduce the occurrence of inbreeding in black grouse (Tetrao tetrix). Mol Ecol 19:1929–1939
Lewin R (1989) Inbreeding costs swamp benefits. Science 243:482
Mabry KE, Shelley EL, Davis KE, Blumstein DT, Van Vuren DH (2013) Social mating system and sex-biased dispersal in mammals and birds: a phylogenetic analysis. PLoS One 8:e57980
Massaro M, Sainudiin R, Merton D, Briskie JV, Poole AM, Hale ML (2013a) Human-assisted spread of a maladaptive behavior in a critically endangered bird. PLoS One 8:e79066
Massaro M, Stanbury M, Briskie JV (2013b) Nest site selection by the endangered black robin increases vulnerability to predation by an invasive bird. Anim Conserv 16:404–411
Matthysen E (2005) Density-dependent dispersal in birds and mammals. Ecography 28:403–416
Moore J, Ali R (1984) Are dispersal and inbreeding avoidance related? Anim Behav 32:94–112
O’Grady JJ, Brook BW, Reed DH, Ballou JD, Tonkyn DW, Frankham R (2006) Realistic levels of inbreeding depression strongly affect extinction risk in wild populations. Biol Conserv 133:42–51
Perrin N, Mazalov V (1999) Dispersal and inbreeding avoidance. Am Nat 154:282–292
Pusey AE (1987) Sex-biased dispersal and inbreeding avoidance in birds and mammals. Trends Ecol Evol 2:295–299
Pusey A, Wolf M (1996) Inbreeding avoidance in animals. Trends Ecol Evol 11:201–206
R Core Team (2016) R: a language and environment for statistical computing, 3.3.1 edn. R Foundation for Statistical Computing, Vienna, Austria, https://www.r-project.org/
Ronce O (2007) How does it feel to be like a rolling stone? Ten questions about dispersal evolution. Annu Rev Ecol Evol S 38:231–253
Stephens PA, Sutherland WJ (1999) Consequences of the Allee effect for behaviour, ecology and conservation. Trends Ecol Evol 14:401–405
Valcu M, Kempenaers B (2008) Causes and consequences of breeding dispersal and divorce in a blue tit, Cyanistes caeruleus, population. Anim Behav 75:1949–1963
Waser PM, Jones WT (1983) Natal philopatry among solitary mammals. Q Rev Biol 58:355–390
Weiser EL, Grueber CE, Kennedy ES, Jamieson IG (2016) Unexpected positive and negative effects of continuing inbreeding in one of the world’s most inbred wild animals. Evolution 70:154–166
Wheelwright NT, Mauck RA (1998) Philopatry, natal dispersal, and inbreeding avoidance in an island population of savannah sparrows. Ecology 79:755–767
Acknowledgments
This research was only possible with permission from the Chatham Island Conservation Board and the logistic help of the Chatham Islands Area office of the Department of Conservation. We especially thank D. Houston, A. Leseberg, A. Liddy, J. Clarkson, K. Hunt, and T. Bliss from the Department of Conservation. S. Allen, R. Bishop, A. Botha, A. Church, K. Drew, S. Fern, N. Green, B. Hunter, B. Kurenbach, H. Lange, J. Muir, L. Poulson, A. Sides, B. Smith, M. Stanbury, A. Wagenhoff, A. Walleyn, M. Walters, and C. Wickes assisted with data collection in the 2007–2011 seasons. We also thank two anonymous reviewers for insightful comments. All research was conducted with approval from the Animal Ethics Committee at the University of Canterbury (permit numbers 2008/18R and 2011/15R) and the New Zealand Department of Conservation.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
Melanie Massaro has received research funding for this project by the New Zealand Foundation for Research, Science and Technology (UOCX0601) for work undertaken in 2007–2009, by the School of Biological Sciences, University of Canterbury, for work in 2010, and by a grant from the Brian Mason Scientific and Technical Trust in 2011.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Communicated by C. R. Brown
Electronic supplementary material
ESM 1
(DOC 153 kb)
Rights and permissions
About this article
Cite this article
Paris, D., Nicholls, A.O., Hall, A. et al. Female-biased dispersal in a spatially restricted endemic island bird. Behav Ecol Sociobiol 70, 2061–2069 (2016). https://doi.org/10.1007/s00265-016-2210-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00265-016-2210-3