Abstract
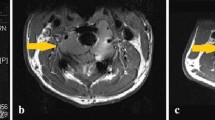
Recent clinical studies have suggested that denosumab is associated with beneficial tumour response, surgical down-staging, and reduced surgical morbidity in patients with giant cell tumour of bone. However, these studies reported results of patients still on denosumab treatment, or patients after denosumab treatment but with a short follow-up. Other studies reported that the new osseous tumour matrix and thickened cortical bone that develop with denosumab treatment does not allow the surgeon to delineate the true extent of the tumour, and probably increases the risk for local recurrence. A study showed that cell proliferation is only diminished by denosumab; the cells continue to proliferate in vitro, albeit at a slower rate. More importantly, nine cases of malignant transformation of GCT during denosumab therapy without previous radiation exposure have been reported; inhibition of RANKL may increase the risk of new malignancies due to immunosuppression. With these concerns in mind, this article is an attempt to put essential information in one place, creating a comprehensive review that the curious reader would find interesting and informative.
Similar content being viewed by others
References
Klenke FM, Wenger DE, Inwards CY et al (2011) Giant cell tumor of bone: risk factors for recurrence. Clin Orthop 469:591–599. doi:10.1007/s11999-010-1501-7
Errani C, Ruggieri P, Asenzio MAN et al (2010) Giant cell tumor of the extremity: a review of 349 cases from a single institution. Cancer Treat Rev 36:1–7. doi:10.1016/j.ctrv.2009.09.002
van der Heijden L, Dijkstra PDS, van de Sande MAJ et al (2014) The clinical approach toward giant cell tumor of bone. Oncologist 19:550–561. doi:10.1634/theoncologist.2013-0432
Balke M, Schremper L, Gebert C et al (2008) Giant cell tumor of bone: treatment and outcome of 214 cases. J Cancer Res Clin Oncol 134:969–978. doi:10.1007/s00432-008-0370-x
Kivioja AH, Blomqvist C, Hietaniemi K et al (2008) Cement is recommended in intralesional surgery of giant cell tumors: a Scandinavian sarcoma group study of 294 patients followed for a median time of 5 years. Acta Orthop 79:86–93. doi:10.1080/17453670710014815
Lackman RD, Crawford EA, King JJ, Ogilvie CM (2009) Conservative treatment of Campanacci grade III proximal humerus giant cell tumors. Clin Orthop 467:1355–1359. doi:10.1007/s11999-008-0583-y
Prosser GH, Baloch KG, Tillman RM et al (2005) Does curettage without adjuvant therapy provide low recurrence rates in giant-cell tumors of bone? Clin Orthop:211–218
Turcotte RE, Wunder JS, Isler MH et al (2002) Giant cell tumor of long bone: a Canadian sarcoma group study. Clin Orthop:248–258
Gaston CL, Grimer RJ, Parry M et al (2016) Current status and unanswered questions on the use of Denosumab in giant cell tumor of bone. Clin Sarcoma Res 6:15. doi:10.1186/s13569-016-0056-0
Thomas D, Henshaw R, Skubitz K et al (2010) Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study. Lancet Oncol 11:275–280. doi:10.1016/S1470-2045(10)70010-3
Chawla S, Henshaw R, Seeger L et al (2013) Safety and efficacy of denosumab for adults and skeletally mature adolescents with giant cell tumour of bone: interim analysis of an open-label, parallel-group, phase 2 study. Lancet Oncol 14:901–908. doi:10.1016/S1470-2045(13)70277-8
Ueda T, Morioka H, Nishida Y et al (2015) Objective tumor response to denosumab in patients with giant cell tumor of bone: a multicenter phase II trial. Ann Oncol 26:2149–2154. doi:10.1093/annonc/mdv307
Rutkowski P, Ferrari S, Grimer RJ et al (2015) Surgical downstaging in an open-label phase II trial of denosumab in patients with giant cell tumor of bone. Ann Surg Oncol 22:2860–2868. doi:10.1245/s10434-015-4634-9
Traub F, Singh J, Dickson BC et al (2016) Efficacy of denosumab in joint preservation for patients with giant cell tumour of the bone. Eur J Cancer 59:1–12. doi:10.1016/j.ejca.2016.01.006
Müller DA, Beltrami G, Scoccianti G et al (2016) Risks and benefits of combining denosumab and surgery in giant cell tumor of bone-a case series. World J Surg Oncol 14:281. doi:10.1186/s12957-016-1034-y
Rekhi B, Verma V, Gulia A et al (2016) Clinicopathological features of a series of 27 cases of post-denosumab treated giant cell tumors of bones: a single institutional experience at a tertiary cancer referral centre. Pathol Oncol Res 23:157-164. doi:10.1007/s12253-016-0123-0
Goldschlager T, Dea N, Boyd M et al (2015) Giant cell tumors of the spine: has denosumab changed the treatment paradigm? J Neurosurg Spine 22:526–533. doi:10.3171/2014.10.SPINE13937
de Carvalho Cavalcante RA, Silva Marques RA, dos Santos VG et al (2016) Spondylectomy for giant cell tumor after denosumab therapy. Spine 41:E178–E182. doi:10.1097/BRS.0000000000001191
Mak IWY, Evaniew N, Popovic S et al (2014) A translational study of the neoplastic cells of giant cell tumor of bone following neoadjuvant denosumab. J Bone Joint Surg Am 96:e127. doi:10.2106/JBJS.M.01332
Aponte-Tinao LA, Piuzzi NS, Roitman P, Farfalli GL (2015) A high-grade sarcoma arising in a patient with recurrent benign giant cell tumor of the proximal tibia while receiving treatment with denosumab. Clin Orthop 473:3050–3055. doi:10.1007/s11999-015-4249-2
Broehm CJ, Garbrecht EL, Wood J, Bocklage T (2015) Two cases of sarcoma arising in giant cell tumor of bone treated with denosumab. Case Rep Med 2015:767198. doi:10.1155/2015/767198
Criscitiello C, Viale G, Gelao L et al (2015) Crosstalk between bone niche and immune system: osteoimmunology signaling as a potential target for cancer treatment. Cancer Treat Rev 41:61–68. doi:10.1016/j.ctrv.2014.12.001
Smith MR, Egerdie B, Hernández Toriz N et al (2009) Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med 361:745–755. doi:10.1056/NEJMoa0809003
Ellis GK, Bone HG, Chlebowski R et al (2008) Randomized trial of denosumab in patients receiving adjuvant aromatase inhibitors for nonmetastatic breast cancer. J Clin Oncol 26:4875–4882. doi:10.1200/JCO.2008.16.3832
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
No benefits have been or will be received from a commercial party related directed or indirectly to the subject matter of this article.
Funding
There is no funding source.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Errani, C., Tsukamoto, S. & Mavrogenis, A.F. How safe and effective is denosumab for bone giant cell tumour?. International Orthopaedics (SICOT) 41, 2397–2400 (2017). https://doi.org/10.1007/s00264-017-3536-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00264-017-3536-9