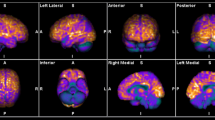
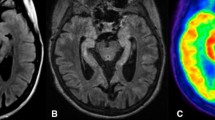
Abstract
Objective
To consolidate current understanding of detection sensitivity of brain 18F-FDG PET scans in the diagnosis of autoimmune encephalitis and to define specific metabolic imaging patterns for the most frequently occurring autoantibodies.
Methods
A systematic and exhaustive search of data available in the literature was performed by querying the PubMed/MEDLINE and Cochrane databases for the search terms: ((PET) OR (positron emission tomography)) AND ((FDG) OR (fluorodeoxyglucose)) AND ((encephalitis) OR (brain inflammation)). Studies had to satisfy the following criteria: (i) include at least ten pediatric or adult patients suspected or diagnosed with autoimmune encephalitis according to the current recommendations, (ii) specifically present 18F-FDG PET and/or morphologic imaging findings. The diagnostic 18F-FDG PET detection sensitivity in autoimmune encephalitis was determined for all cases reported in this systematic review, according to a meta-analysis following the PRISMA method, and selected publication quality was assessed with the QUADAS-2 tool.
Results
The search strategy identified 626 articles including references from publications. The detection sensitivity of 18F-FDG PET was 87% (80–92%) based on 21 publications and 444 patients included in the meta-analysis. We also report specific brain 18F-FDG PET imaging patterns for the main encephalitis autoantibody subtypes.
Conclusion and relevance
Brain 18F-FDG PET has a high detection sensitivity and should be included in future diagnostic autoimmune encephalitis recommendations. Specific metabolic 18F-FDG PET patterns corresponding to the main autoimmune encephalitis autoantibody subtypes further enhance the value of this diagnostic.



Similar content being viewed by others
References
Graus F, Titulaer MJ, Balu R, et al. A clinical approach to diagnosis of autoimmune encephalitis. Lancet Neurol. 2016;15(4):391–404. https://doi.org/10.1016/S1474-4422(15)00401-9.
Jmor F, Emsley HC, Fischer M, Solomon T, Lewthwaite P. The incidence of acute encephalitis syndrome in Western industrialised and tropical countries. Virol J. 2008;5(1):134. https://doi.org/10.1186/1743-422X-5-134.
Titulaer MJ, McCracken L, Gabilondo I, et al. Treatment and prognostic factors for long-term outcome in patients with anti-NMDA receptor encephalitis: an observational cohort study. Lancet Neurol. 2013;12(2):157–65. https://doi.org/10.1016/S1474-4422(12)70310-1.
Granerod J, Ambrose HE, Davies NW, et al. Causes of encephalitis and differences in their clinical presentations in England: a multicentre, population-based prospective study. Lancet Infect Dis. 2010;10(12):835–44. https://doi.org/10.1016/S1473-3099(10)70222-X.
Mailles A, Stahl J. Infectious encephalitis in France in 2007: a national prospective study. Clin Infect Dis. 2009;49(12):1838–47. https://doi.org/10.1086/648419.
Cózar Santiago MDP, Sanchez Jurado R, Sanz Llorens R, Aguilar Barrios JE, Ferrer Rebolleda J. Limbic encephalitis diagnosed With 18F-FDG PET/CT. Clin Nucl Med. 2016;41(2):e101–3. https://doi.org/10.1097/RLU.0000000000001076.
Lancaster E, Martinez-Hernandez E, Dalmau J. Encephalitis and antibodies to synaptic and neuronal cell surface proteins. Neurology. 2011;77(2):179–89. https://doi.org/10.1212/WNL.0b013e318224afde.
Rosenfeld MR, Dalmau J. Paraneoplastic neurologic disorders: a brief overview. Memo. 2012;5(3):197–200. https://doi.org/10.1007/s12254-012-0034-z.
McKeon A. Paraneoplastic and other autoimmune disorders of the central nervous system. Neurohospitalist. 2013;3(2):53–64. https://doi.org/10.1177/1941874412453339.
Dutra LA, Abrantes F, Toso FF, Pedroso JL, Barsottini OGP, Hoftberger R. Autoimmune encephalitis: a review of diagnosis and treatment. Arq Neuropsiquiatr. 2018;76(1):41–9. https://doi.org/10.1590/0004-282X20170176.
Darnell RB, Posner JB. Paraneoplastic syndromes involving the nervous system. N Engl J Med. 2003;349(16):1543–54. https://doi.org/10.1056/NEJMra023009.
Armangue T, Leypoldt F, Dalmau J. Autoimmune encephalitis as differential diagnosis of infectious encephalitis. Curr Opin Neurol. 2014;27(3):361–8. https://doi.org/10.1097/WCO.0000000000000087.
Heine J, Prüss H, Bartsch T, Ploner CJ, Paul F, Finke C. Imaging of autoimmune encephalitis--relevance for clinical practice and hippocampal function. Neuroscience. 2015;309:68–83. https://doi.org/10.1016/j.neuroscience.2015.05.037.
Diaz-Arias LA, Pardo CA, Probasco JC. Autoimmune encephalitis in the intensive care unit. In: Nelson SE, Nyquist PA, eds. Neurointensive Care Unit. Current Clinical Neurology. Springer International Publishing; 2020:249–263. https://doi.org/10.1007/978-3-030-36548-6_17.
Guerin J, Watson RE, Carr CM, Liebo GB, Kotsenas AL. Autoimmune epilepsy: findings on MRI and FDG-PET. Br J Radiol. 2019;92(1093). https://doi.org/10.1259/bjr.20170869.
Kyritsis AP, Markoula S, Alexiou G, et al. Diagnosis and treatment of limbic encephalitis in the cancer patient. Future Oncol Published online June 8, 2020. https://doi.org/10.2217/fon-2020-0080.
Wang H, **ao Z. Current progress on assessing the prognosis for anti-N-methyl-D-aspartate receptor (NMDAR) encephalitis. Biomed Res Int. 2020;2020:7506590. https://doi.org/10.1155/2020/7506590.
Husari KS, Dubey D. Autoimmune epilepsy. Neurotherapeutics. 2019;16(3):685–702. https://doi.org/10.1007/s13311-019-00750-3.
Bacchi S, Franke K, Wewegama D, Needham E, Patel S, Menon D. Magnetic resonance imaging and positron emission tomography in anti-NMDA receptor encephalitis: a systematic review. J Clin Neurosci. 2018;52:54–9. https://doi.org/10.1016/j.jocn.2018.03.026.
Dalmau J, Geis C, Graus F. Autoantibodies to synaptic receptors and neuronal cell surface proteins in autoimmune diseases of the central nervous system. Physiol Rev. 2017;97(2):839–87. https://doi.org/10.1152/physrev.00010.2016.
Arbizu J, Giuliani A, Gállego Perez-Larraya J, et al. Emerging clinical issues and multivariate analyses in PET investigations. Q J Nucl Med Mol Imaging. 2017;61(4):386–404. https://doi.org/10.23736/S1824-4785.17.03024-2.
Venkatraman A, Opal P. Paraneoplastic cerebellar degeneration with anti-Yo antibodies – a review. Ann Clin Transl Neurol. 2016;3(8):655–63. https://doi.org/10.1002/acn3.328.
Lim M, Gorman M. Autoimmune neurologic disorders in children. Handb Clin Neurol. 2016;133:485–510. https://doi.org/10.1016/B978-0-444-63432-0.00026-8.
Quartuccio N, Caobelli F, Evangelista L, et al. The role of PET/CT in the evaluation of patients affected by limbic encephalitis: a systematic review of the literature. J Neuroimmunol. 2015;284:44–8. https://doi.org/10.1016/j.jneuroim.2015.05.002.
Lee S-W, Park M, Lee S-K, Park Y-B. The efficacy of brain F-fluorodeoxyglucose positron emission tomography in neuropsychiatric lupus patients with normal brain magnetic resonance imaging findings. Lupus. 2012;21(14):1531–7. https://doi.org/10.1177/0961203312459104.
McInnes MDF, Moher D, Thombs BD, et al. Preferred reporting items for a systematic review and meta-analysis of diagnostic test accuracy studies: the PRISMA-DTA statement. JAMA. 2018;319(4):388–96. https://doi.org/10.1001/jama.2017.19163.
Whiting PF, Rutjes AWS, Westwood ME, et al. QUADAS-2: a revised tool for the quality assessment of diagnostic accuracy studies. Ann Intern Med. 2011;155(8):529–36. https://doi.org/10.7326/0003-4819-155-8-201110180-00009.
Kerik-Rotenberg N, Diaz-Meneses I, Hernandez-Ramirez R, et al. A metabolic brain pattern associated with anti-N-methyl-D-aspartate receptor encephalitis. Psychosomatics. 2020;61(1):39–48. https://doi.org/10.1016/j.psym.2019.08.007.
Turpin S, Martineau P, Levasseur M-A, et al. 18F-Flurodeoxyglucose positron emission tomography with computed tomography (FDG PET/CT) findings in children with encephalitis and comparison to conventional imaging. Eur J Nucl Med Mol Imaging. 2019;46(6):1309–24. https://doi.org/10.1007/s00259-019-04302-x.
Schubert J, Brämer D, Huttner HB, et al. Management and prognostic markers in patients with autoimmune encephalitis requiring ICU treatment. Neurol Neuroimmunol Neuroinflamm. 2018;6(1). https://doi.org/10.1212/NXI.0000000000000514.
Probasco JC, Solnes L, Nalluri A, et al. Abnormal brain metabolism on FDG-PET/CT is a common early finding in autoimmune encephalitis. Neurol Neuroimmunol Neuroinflamm. 2017;4(4):e352. https://doi.org/10.1212/NXI.0000000000000352.
Fiorella DJ, Provenzale JM, Coleman RE, Crain BJ, Al-Sugair AA. (18)F-fluorodeoxyglucose positron emission tomography and MR imaging findings in Rasmussen encephalitis. AJNR Am J Neuroradiol. 2001;22(7):1291–9.
Malter MP, Helmstaedter C, Urbach H, Vincent A, Bien CG. Antibodies to glutamic acid decarboxylase define a form of limbic encephalitis. Ann Neurol. 2010;67(4):470–8. https://doi.org/10.1002/ana.21917.
Aupy J, Collongues N, Blanc F, Tranchant C, Hirsch E, De Seze J. Encéphalites dysimmunitaires, données cliniques, radiologiques et immunologiques. Rev Neurol. 2013;169(2):142–53. https://doi.org/10.1016/j.neurol.2012.05.014.
Baumgartner A, Rauer S, Mader I, Meyer PT. Cerebral FDG-PET and MRI findings in autoimmune limbic encephalitis: correlation with autoantibody types. J Neurol. 2013;260(11):2744–53. https://doi.org/10.1007/s00415-013-7048-2.
Shin Y-W, Lee S-T, Shin J-W, et al. VGKC-complex/LGI1-antibody encephalitis: clinical manifestations and response to immunotherapy. J Neuroimmunol. 2013;265(1–2):75–81. https://doi.org/10.1016/j.jneuroim.2013.10.005.
Masangkay N, Basu S, Moghbel M, Kwee T, Alavi A. Brain 18F-FDG-PET characteristics in patients with paraneoplastic neurological syndrome and its correlation with clinical and MRI findings. Nucl Med Commun. 2014;35(10):1038–46. https://doi.org/10.1097/MNM.0000000000000163.
Wegner F, Wilke F, Raab P, et al. Anti-leucine rich glioma inactivated 1 protein and anti-N-methyl-D-aspartate receptor encephalitis show distinct patterns of brain glucose metabolism in 18F-fluoro-2-deoxy-d-glucose positron emission tomography. BMC Neurol. 2014;14(1):136. https://doi.org/10.1186/1471-2377-14-136.
Flanagan EP, Kotsenas AL, Britton JW, et al. Basal ganglia T1 hyperintensity in LGI1-autoantibody faciobrachial dystonic seizures. Neurol Neuroimmunol Neuroinflamm. 2015;2(6). https://doi.org/10.1212/NXI.0000000000000161.
Solnes LB, Jones KM, Rowe SP, et al. Diagnostic value of 18 F-FDG PET/CT versus MRI in the setting of antibody-specific autoimmune encephalitis. J Nucl Med. 2017;58(8):1307–13. https://doi.org/10.2967/jnumed.116.184333.
Jang Y, Lee S-T, Bae J-Y, et al. LGI1 expression and human brain asymmetry: insights from patients with LGI1-antibody encephalitis. J Neuroinflammation. 2018;15. https://doi.org/10.1186/s12974-018-1314-2.
Steriade C, Moosa ANV, Hantus S, Prayson RA, Alexopoulos A, Rae-Grant A. Electroclinical features of seizures associated with autoimmune encephalitis. Seizure. 2018;60:198–204. https://doi.org/10.1016/j.seizure.2018.06.021.
Tripathi M, Tripathi M, Roy SG, et al. Metabolic topography of autoimmune non-paraneoplastic encephalitis. Neuroradiology. 2018;60(2):189–98. https://doi.org/10.1007/s00234-017-1956-2.
Falip M, Rodriguez-Bel L, Castañer S, et al. Hippocampus and insula are targets in epileptic patients with glutamic acid decarboxylase antibodies. Front Neurol. 2018;9:1143. https://doi.org/10.3389/fneur.2018.01143.
Lv R-J, Pan J, Zhou G, et al. Semi-quantitative FDG-PET analysis increases the sensitivity compared with visual analysis in the diagnosis of autoimmune encephalitis. Front Neurol. 2019;10:576. https://doi.org/10.3389/fneur.2019.00576.
Strohm T, Steriade C, Wu G, Hantus S, Rae-Grant A, Larvie M. FDG-PET and MRI in the evolution of new-onset refractory status Epilepticus. Am J Neuroradiol. 2019;40(2):238–44. https://doi.org/10.3174/ajnr.A5929.
Deuschl C, Rüber T, Ernst L, et al. 18F-FDG-PET/MRI in the diagnostic work-up of limbic encephalitis. Treglia G, ed. PLoS One. 2020;15(1):e0227906. https://doi.org/10.1371/journal.pone.0227906.
Liu X, Shan W, Zhao X, et al. The clinical value of 18F-FDG-PET in autoimmune encephalitis associated with LGI1 antibody. Front Neurol. 2020;11:418. https://doi.org/10.3389/fneur.2020.00418.
Graus F. Recommended diagnostic criteria for paraneoplastic neurological syndromes. J Neurol Neurosurg Psychiatry. 2004;75(8):1135–40. https://doi.org/10.1136/jnnp.2003.034447.
Venkatesan A, Adatia K. Anti-NMDA-receptor encephalitis: from bench to clinic. ACS Chem Neurosci. 2017;8(12):2586–95. https://doi.org/10.1021/acschemneuro.7b00319.
Leypoldt F, Armangue T, Dalmau J. Autoimmune encephalopathies: autoimmune encephalopathies. Ann N Y Acad Sci. 2015;1338(1):94–114. https://doi.org/10.1111/nyas.12553.
Najjar S, Steiner J, Najjar A, Bechter K. A clinical approach to new-onset psychosis associated with immune dysregulation: the concept of autoimmune psychosis. J Neuroinflammation. 2018;15. https://doi.org/10.1186/s12974-018-1067-y.
Staley EM, Jamy R, Phan AQ, Figge DA, Pham HP. N -methyl- D -aspartate receptor antibody encephalitis: a concise review of the disorder, diagnosis, and management. ACS Chem Neurosci. 2019;10(1):132–42. https://doi.org/10.1021/acschemneuro.8b00304.
Probasco JC, Solnes L, Nalluri A, et al. Decreased occipital lobe metabolism by FDG-PET/CT: an anti-NMDA receptor encephalitis biomarker. Neurol Neuroimmunol Neuroinflamm. 2018;5(1):e413. https://doi.org/10.1212/NXI.0000000000000413.
Goudot M, Frismand S, Hopes L, et al. GAD65-Ab encephalitis and subtle focal status epilepticus. Epileptic Disord. 2019;21(5):437–42. https://doi.org/10.1684/epd.2019.1094.
Varadkar S, Bien CG, Kruse CA, et al. Rasmussen’s encephalitis: clinical features, pathobiology, and treatment advances. Lancet Neurol. 2014;13(2):195–205. https://doi.org/10.1016/S1474-4422(13)70260-6.
Dalmau J, Lancaster E, Martinez-Hernandez E, Rosenfeld MR, Balice-Gordon R. Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurol. 2011;10(1):63–74. https://doi.org/10.1016/S1474-4422(10)70253-2.
Nobili F, Arbizu J, Bouwman F, et al. European Association of Nuclear Medicine and European Academy of Neurology recommendations for the use of brain 18 F-fluorodeoxyglucose positron emission tomography in neurodegenerative cognitive impairment and dementia: Delphi consensus. Eur J Neurol. 2018;25(10):1201–17. https://doi.org/10.1111/ene.13728.
Hansen N, Widman G, Stuff S, et al. Cancer frequency detected by positron emission tomography-computed tomography in limbic encephalitis. Epilepsy Behav. 2018;89:105–11. https://doi.org/10.1016/j.yebeh.2018.09.043.
Kampe KKW, Rotermund R, Tienken M, et al. Diagnostic value of positron emission tomography combined with computed tomography for evaluating critically ill neurological patients. Front Neurol. 2017;8. https://doi.org/10.3389/fneur.2017.00033.
Leypoldt F, Buchert R, Kleiter I, et al. Fluorodeoxyglucose positron emission tomography in anti-N-methyl-D-aspartate receptor encephalitis: distinct pattern of disease. J Neurol Neurosurg Psychiatry. 2012;83(7):681–6. https://doi.org/10.1136/jnnp-2011-301969.
Verger A, Lagarde S, Maillard L, Bartolomei F, Guedj E. Brain molecular imaging in pharmacoresistant focal epilepsy: current practice and perspectives. Rev Neurol (Paris). Published online June 5, 2017. https://doi.org/10.1016/j.neurol.2017.05.001.
Availability of data and material
The data that support the findings of this study are available on request from the corresponding author (AV).
Code availability
Not applicable.
Author information
Authors and Affiliations
Contributions
All authors contributed significantly to the analysis and interpretation of the data (MB, MD, MBC, AV), to the writing of the manuscript (EM, AV) and to the revision of the manuscript (EG, AK, LT, AV).
Corresponding author
Ethics declarations
Ethics approval and consent to participate
This article does not contain any studies with human participants or animals performed by any of the authors.
Consent for publication
The consent has been obtained for each patient for whom their FDG PET images are included in the manuscript.
Conflicts of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Neurology
Supplementary information
ESM 1
(DOCX 1097 kb)
Rights and permissions
About this article
Cite this article
Bordonne, M., Chawki, M.B., Doyen, M. et al. Brain 18F-FDG PET for the diagnosis of autoimmune encephalitis: a systematic review and a meta-analysis. Eur J Nucl Med Mol Imaging 48, 3847–3858 (2021). https://doi.org/10.1007/s00259-021-05299-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-021-05299-y