Abstract
Purpose
18F-FDG PET is routinely used as an imaging marker in the early and differential diagnosis of dementing disorders and has incremental value over the clinical neurological and neuropsychological evaluation. Perfusion MR imaging by means of arterial spin labelling (ASL) is an alternative modality to indirectly measure neuronal functioning and could be used as complement measurement in a single MR session in the workup of dementia. Using simultaneous PET-MR, we performed a direct head-to-head comparison between enhanced multiplane tagging ASL (eASL) and 18F-FDG PET in a true clinical context of subjects referred for suspicion of neurodegenerative dementia.
Methods
Twenty-seven patients underwent a 20-min 18F-FDG PET/MR and simultaneously acquired eASL on a GE Signa PET/MR. Data were compared with 30 screened age- and gender-matched healthy controls. Both integral eASL and 18F-FDG datasets were analysed visually by two readers unaware of the final clinical diagnosis, either in normal/abnormal classes, or full differential diagnosis (normal, Alzheimer type dementia [AD], dementia with Lewy Bodies [LBD], frontotemporal dementia [FTD] or other). Reader confidence was assessed with a rating scale (range 1–4). Data were also analysed semiquantitatively by VOI and voxel-based analyses.
Results
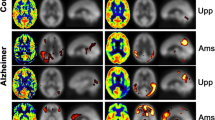
The ground truth diagnosis for the patient group resulted in 14 patients with a neurodegenerative cognitive disorder (AD, FTD, LBD) and 13 patients with no arguments for an underlying neurodegenerative cause. Visual analysis resulted in equal specificity (0.70) for differentiating normal and abnormal cases between the two modalities, but in a higher sensitivity (0.93), confidence rating (0.64) and interobserver agreement for 18F-FDG PET compared with eASL. The same was true for assigning a specific differential diagnosis (sensitivity: and 0.39 for 18F-FDG PET and eASL, respectively). Semiquantitative analyses revealed prototypical patterns for AD and FTD, with both higher volumes of abnormality and intensity differences on 18F-FDG PET.
Conclusion
In a direct head-to-head comparison on a simultaneous GE Signa PET/MR, 18F-FDG PET performed better compared with ASL in terms of sensitivity and reader confidence, as well as volume and intensity of abnormalities. However, using pure semiquantitative analysis, similar diagnostic accuracy between the two modalities was obtained. Therefore, ASL may still serve as complement to neuroreceptor or protein deposition PET studies when a single simultaneous investigation is warranted.






Similar content being viewed by others
References
Drew L. An age-old story of dementia. Nature. 2018;559:S2–3.
Prince M, Bryce R, Albanese E, Wimo A, Ribeiro W, Ferri CP. The global prevalence of dementia: a systematic review and metaanalysis. Alzheimer’s Dement. 2013;9:63–75.e2.
Shivamurthy VK, Tahari AK, Marcus C, Subramaniam RM, Vkn S. Brain FDG PET and the diagnosis of dementia.
Amy Orciari Herman PFW. For patients with cognitive impairment, amyloid PET leads to changes in clinical management. NEJM J Watch. 2019;2019.
Villemagne VL, Doré V, Bourgeat P, Burnham SC, Laws S, Salvado O, et al. Aβ-amyloid and tau imaging in dementia. Semin Nucl Med. 2017;47:75–88.
Okamura N, Harada R, Furumoto S, Tago T, Yanai K, Arai H, et al. Advances in the development of tau PET radiotracers and their clinical applications. Ageing Res Rev. 2016;30:107–13.
Mallik A, Drzezga A, Minoshima S. Clinical amyloid imaging. Semin Nucl Med. 2017;47:31–43.
Morbelli S, Garibotto V, Van De Giessen E, Arbizu J, Chételat G, Drezgza A, et al. A Cochrane review on brain [18F]FDG PET in dementia: limitations and future perspectives. Eur J Nucl Med Mol Imaging. 2015;42:1487–91.
Garibotto V, Herholz K, Boccardi M, Picco A, Varrone A, Nordberg A, et al. Clinical validity of brain fluorodeoxyglucose positron emission tomography as a biomarker for Alzheimer’s disease in the context of a structured 5-phase development framework. Neurobiol Aging. 2017;52:183–95.
Foster NL, Heidebrink JL, Clark CM, Jagust WJ, Arnold SE, Barbas NR, et al. FDG-PET improves accuracy in distinguishing frontotemporal dementia and Alzheimer’s disease. Brain. 2007;130:2616–35.
Tosun D, Schuff N, Rabinovici GD, Ayakta N, Miller BL, Jagust W, et al. Diagnostic utility of ASL-MRI and FDG-PET in the behavioral variant of FTD and AD. Ann Clin Transl Neurol. 2016;3:740–51.
Jueptner M, Weiller C. Review: does measurement of regional cerebral blood flow reflect synaptic activity? Implications for PET and fMRI. Neuroimage. 1995;2:148–56.
Du AT, Jahng GH, Hayasaka S, Kramer JH, Rosen HJ, Gorno-Tempini ML, et al. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67:1215–20.
Raji CA, Lee C, Lopez OL, Tsay J, Boardman JF, Schwartz ED, et al. Initial experience in using continuous arterial spin-labeled MR imaging for early detection of Alzheimer disease. AJNR Am J Neuroradiol. 2010;31:847–55.
Kaneta T, Katsuse O, Hirano T, Ogawa M, Shihikura-Hino A, Yoshida K, et al. Voxel-wise correlations between cognition and cerebral blood flow using arterial spin-labeled perfusion MRI in patients with Alzheimer’s disease: a cross-sectional study. BMC Neurol. 2017;17:91.
Liu P, Uh J, Devous MD, Adinoff B, Lu H. Comparison of relative cerebral blood flow maps using pseudo-continuous arterial spin labeling and single photon emission computed tomography. NMR Biomed. 2012;25:779–86.
Fällmar D, Haller S, Lilja J, Danfors T, Kilander L, Tolboom N, et al. Arterial spin labeling-based Z-maps have high specificity and positive predictive value for neurodegenerative dementia compared to FDG-PET. Eur Radiol. 2017;27:4237–46.
Verfaillie SCJ, Adriaanse SM, Binnewijzend MAA, Benedictus MR, Ossenkoppele R, Wattjes MP, et al. Cerebral perfusion and glucose metabolism in Alzheimer’s disease and frontotemporal dementia: two sides of the same coin? Eur Radiol. 2015;25:3050–9.
Anazodo UC, Finger E, Kwan BYM, Pavlosky W, Warrington JC, Günther M, et al. Using simultaneous PET/MRI to compare the accuracy of diagnosing frontotemporal dementia by arterial spin labelling MRI and FDG-PET. Neuro Image Clin. 2018;17:405–14.
Riederer I, Bohn KP, Preibisch C, Wiedemann E, Zimmer C, Alexopoulos P, et al. Alzheimer disease and mild cognitive impairment: integrated pulsed arterial spin-labeling MRI and 18 F-FDG PET. Radiology. 2018;288:198–206.
Weyts K, Vernooij M, Steketee R, Valkema R, Smits M. Qualitative agreement and diagnostic performance of arterial spin labelling MRI and FDG PET-CT in suspected early-stage dementia: comparison of arterial spin labelling MRI and FDG PET-CT in suspected dementia. Clin Imaging. 2017;45:1–7.
Musiek ES, Chen Y, Korczykowski M, Saboury B, Martinez PM, Reddin JS, et al. Direct comparison of FDG-PET and ASL-MRI in Alzheimer’s disease. Alzheimers Dement. 2012;8:51–9.
Douglas D, Goubran M, Wilson E, Xu G, Tripathi P, Holley D, et al. Correlation between arterial spin labeling MRI and dynamic FDG on PET-MR in Alzheimer’s disease and non-Alzhiemer’s disease patients. EJNMMI Phys. 2015;2:A83.
Hammers A, Allom R, Koepp MJ, Free SL, Myers R, Lemieux L, et al. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum Brain Mapp. 2003;19:224–47.
Bohnen NI, Djang DSW, Herholz K, Anzai Y, Minoshima S. Effectiveness and safety of 18F-FDG PET in the evaluation of dementia: a review of the recent literature. J Nucl Med. 2012;53:59–71.
Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, part 1: technique and artifacts. AJNR Am J Neuroradiol. 2008;29:1228–34.
Grade M, Hernandez Tamames JA, Pizzini FB, Achten E, Golay X, Smits M. A neuroradiologist’s guide to arterial spin labeling MRI in clinical practice. Neuroradiology. 2015;57:1181–202.
Yoshiura T, Hiwatashi A, Yamashita K, Ohyagi Y, Monji A, Takayama Y, et al. Simultaneous measurement of arterial transit time, arterial blood volume, and cerebral blood flow using arterial spin-labeling in patients with Alzheimer disease. Am J Neuroradiol. 2009;30:1388–93.
Tang X, Cai F, Ding D-X, Zhang L-L, Cai X-Y, Fang Q. Magnetic resonance imaging relaxation time in Alzheimer’s disease. Brain Res Bull. 2018;140:176–89.
Chen Y, Wolk DA, Reddin JS, Korczykowski M, Martinez PM, Musiek ES, et al. Voxel-level comparison of arterial spin-labeled perfusion MRI and FDG-PET in Alzheimer disease. Neurology. 2011;77:1977–85.
Drzezga A, Lautenschlager N, Siebner H, Riemenschneider M, Willoch F, Minoshima S, et al. Cerebral metabolic changes accompanying conversion of mild cognitive impairment into Alzheimer’s disease: a PET follow-up study. Eur J Nucl Med Mol Imaging. 2003;30:1104–13.
Fällmar D, Lilja J, Velickaite V, Danfors T, Lubberink M, Ahlgren A, et al. Visual assessment of brain perfusion MRI scans in dementia: a pilot study. J Neuroimaging. 2016;26:324–30.
Verclytte S, Lopes R, Lenfant P, Rollin A, Semah F, Leclerc X, et al. Cerebral hypoperfusion and hypometabolism detected by arterial spin labeling MRI and FDG-PET in early-onset Alzheimer’s disease. J Neuroimaging. 2016;26:207–12.
Teune LK, Renken Phd RJ, De Jong BM, Willemsen AT, Van Osch MJ, Roerdink JBTM, et al. Parkinson’s disease-related perfusion and glucose metabolic brain patterns identified with PCASL-MRI and FDG-PET imaging. 2014;
Rabinovici GD, Gatsonis C, Apgar C, Chaudhary K, Gareen I, Hanna L, et al. Association of amyloid positron emission tomography with subsequent change in clinical management among Medicare beneficiaries with mild cognitive impairment or dementia. JAMA. 2019;321:1286.
Henriksen OM, Larsson HBW, Hansen AE, Grüner JM, Law I, Rostrup E. Estimation of intersubject variability of cerebral blood flow measurements using MRI and positron emission tomography. J Magn Reson Imaging. 2012;35:1290–9.
Schramm G, Koole M, Willekens SMA, Rezaei A, Van Weehaeghe D, Delso G, et al. Regional accuracy of ZTE-based attenuation correction in static and dynamic brain PET/MR. Med Phys. 2018.
Ferreira LK, Diniz BS, Forlenza OV, Busatto GF, Zanetti MV. Neurostructural predictors of Alzheimer’s disease: a meta-analysis of VBM studies. Neurobiol Aging. 2011;32:1733–41.
Acknowledgements
The authors thank all the participants for their willingness to participate in this study, the PET radiopharmacy, research technologists (Kwinten Porters and Jef Van Loock) and radiology team of UZ Leuven for their skilled support. Jenny Ceccarini and Donatienne Van Weehaeghe are postdoctoral and Ph.D. fellows of the Research Foundation Flanders (FWO), respectively. Rik Vandenberghe, Mathieu Vandenbulcke and Koen Van Laere are Senior Clinical Investigators of the FWO.
Funding
Jenny Ceccarini and Donatienne Van Weehaeghe are postdoctoral and Ph.D. fellows of the Research Foundation Flanders (FWO), respectively. Rik Vandenberghe and Koen Van Laere are Senior Clinical Investigators of the FWO.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jenny Ceccarini and Sophie Bourgeois are shared first authors.
This article is part of the Topical Collection on Neurology
Electronic supplementary material
ESM 1
(DOCX 464 kb)
Rights and permissions
About this article
Cite this article
Ceccarini, J., Bourgeois, S., Van Weehaeghe, D. et al. Direct prospective comparison of 18F-FDG PET and arterial spin labelling MR using simultaneous PET/MR in patients referred for diagnosis of dementia. Eur J Nucl Med Mol Imaging 47, 2142–2154 (2020). https://doi.org/10.1007/s00259-020-04694-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00259-020-04694-1