Abstract
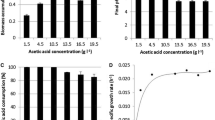
A bacterium classified as Rhodococcus opacus, which is able to use pyridine (a potentially growth-inhibiting substrate) as its sole source of carbon, energy and nitrogen, was isolated. In a carbon-limited chemostat culture, the kinetics was determined for growth on both pyridine and a mixture of pyridine and fructose (9 mM/22.15 mM). With growth on pyridine, stable steady states were achieved up to dilution rates of about 0.1 h−1. A further increase in the dilution rate resulted in the progressive accumulation of pyridine in the culture liquid and the cells were washed out. The maximum specific growth rate (μmax = 0.23 h−1) and the KS value (0.22 mM) for growth on pyridine were determined from the residual pyridine concentrations measured within the range of stable steady states. With growth on the substrate mixture, the specific pyridine consumption rates and the residual pyridine concentrations were lower at similar dilution rates than with growth on pyridine alone, and stable steady states were established at dilution rates of up to 0.13 h−1. The maximum pyridine degradation rate was enhanced to 270 mg pyridine l−1 h−1 compared to 210 mg pyridine l−1 h−1 with growth on pyridine as a single substrate. An external nitrogen source did not need to be added in the case of growth on the substrate mixture. Fructose was assimilated by means of ammonium released from pyridine. Analysis of the nitrogen balance furnished proof that pyridine is an energy-deficient substrate; pyridine was assimilated and dissimilated at a ratio of 1 mol/0.67 mol respectively. The resulting yield coefficient was about 0.55 g dry weight/g pyridine. Moreover, it was demonstrated that, in regard to the biologically usable energy, 1 mol pyridine corresponds to 0.43 mol fructose.
Similar content being viewed by others
References
Anthony C (1978) The prediction of growth yields in methylotrophs. J Gen Microbiol 104: 91–104
Babel W, Müller RH (1985) Correlation between cell composition and carbon conversion efficiency in microbial growth: a theoretical study. Appl Microbiol Biotechnol 22: 201–207
Babel W, Brinkmann U, Müller RH (1993) The auxiliary substrate concept — an approach for overcoming limits of microbial performances. Acta Biotechnol 13: 211–242
Bernt E, Bergmeyer HU (1970) D-Fructose. In: Bergmeyer HU (ed) Methoden der enzymatischen Analyse, vol II. Akademie-Verlag, Berlin, pp 1266–1269
Brinkmann U, Babel W (1992) Simultaneous utilization of heterotrophic substrates by Hansenula polymorpha MH30 results in enhanced growth rates. Appl Microbiol Biotechnol 37: 98–103
Brinkmann U, Müller RH, Babel W (1990) The growth rate-limiting reaction in methanol-assimilating yeasts. FEMS Microbiol Rev 87: 261–265
Dijken JP van, Harder W (1975) Growth yields of microorganisms on methanol and methane. A theoretical study. Biotechnol Bioeng 17: 15–30
Eggeling L, Sahm H (1981) Enhanced utilization-rate of methanol during growth on a mixed substrate: a continuous culture study with Hansenula polymorpha. Arch Microbiol 130: 362–365
Egli T, Lindley ND, Quale JR (1983) Regulation of enzyme synthesis and variation of residual methanol concentration during carbon-limited growth of Kloeckera sp. 2201 on mixtures of methanol and glucose. J Gen Microbiol 129: 1269–1281
Egli T, Bossard C, Hamer G (1986) Simultaneous utilization of methanol/glucose mixtures by Hansenula polymorpha in chemostat: influence of the dilution rate and the mixture composition on the mode of utilization. Biotechnol Bioeng 28: 1735–1741
Ensing JC, Rittenberger SC (1963) A crystalline pigment produced from 2-hydroxypyridine by Arthrobacter crystallopoietes n. sp. Arch Mikrobiol 47: 137–153
Gommers PJF, Schie BJ van, Dijken JP van, Kuenen JG (1988) Biochemical limits to microbial growth yields: an analysis of mixed substrate utilization. Biotechnol Bioeng 32: 86–94
Hauche H, Hilger U (1974) Untersuchungen zur Isolierung und Charakterisierung methanassimilierender Bakterien und zum Einfluß einiger Milieufaktoren auf Wachstum und Zellzusammensetzung. PhD Thesis, Akademie der Wissenschaften, Berlin
Hensel J, Straube G (1990) Kinetic studies of phenol degradation by Rhodococcus sp. P1. II. Continuous cultivation. Antonie van Leeuwenhoek, J Microbiol Ser 57: 33–36
Korosteleva LA, Kost AN, Vorob’eva LI, Modyanova LV, Terent’ev PB, Kulikov NS (1981) Microbiological degradation of pyridine and 3-methylpyridine. Appl Biochem Microbiol 17: 276–28
Shukla OP, Kaul SM (1986) Microbiological transformation of pyridine N-oxide and pyridine by Nocardia sp. Can J Microbiol 32: 330–341
Sims GK, Sommers LE, Konopka A (1986) Degradation of pyridine by Micrococcus luteus isolated from soil. Appl Environ Microbiol 51: 963–968
Watson GK, Cain RB (1975) Microbial metabolism of the pyridine ring. Biochem J 146: 157–172
Zefirov NS, Agapova SR, Terentiev PB, Bulakhova IM, Vasyukova NI, Modyanova LV (1994) Degradation of pyridine by Arthrobacter crystallopoietes and Rhodococcus opacus strains. FEMS Microbiol Lett 118: 71–74
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Brinkmann, U., Babel, W. Simultaneous utilization of pyridine and fructose by Rhodococcus opacus UFZ B 408 without an external nitrogen source. Appl Microbiol Biotechnol 45, 217–223 (1996). https://doi.org/10.1007/s002530050673
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/s002530050673