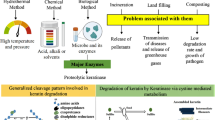
Abstract
Keratinases are well-recognized enzymes with the unique ability to attack highly cross-linked, recalcitrant structural proteins such as keratin. Their potential in environmental clean-up of huge amount of feather waste has been well established since long. Today, they have gained importance in various other biotechnological and pharmaceutical applications. However, commercial availability of keratinases is still limited. Hence, to attract entrepreneurs, investors and enzyme industries it is utmost important to explicitly present the market potential of keratinases through detailed account of its application sectors. Here, the application areas have been divided into three parts: the first one is dealing with the area of exclusive applications, the second emphasizes protease dominated sectors where keratinases would prove better substitutes, and the third deals with upcoming newer areas which still await practical documentation. An account of benefits of keratinase usage, existing market size, and available commercial sources and products has also been presented.
Similar content being viewed by others
References
Aly R (1996) Microbial infections of skin and nails. Medical Microbiol 4th edition. Bookshelf ID:NBK8301
Bartelt-Hunt SL, Bartz JC (2013) Behavior of prions in the environment: Implications for prion biology. PLoS Pathog 9(2):e1003113. doi:10.1371/journal. ppat.1003113
Brandelli A (2008) Bacterial keratinases: useful enzymes for bioprocessing agroindustrial wastes and beyond. Food Bioprocess Technol 1:105–116
Brandelli A, Daroit DJ, Riffel A (2010) Biochemical features of microbial keratinases and their production and applications. Appl Microbial Biotechnol 85:1735–1750
Bressollier P, Letourneau F, Urdaci M, Verneuil B (1999) Purification and characterization of a keratinolytic serine proteinase from Streptomyces albidoflavus. Appl Environ Microbiol 65:2570–2576
Cafe MB, Borges CA, Fritts CA, Waldroup PW (2002) Avizyme improves performance of broilers fed corn-soybean meal-based diets. J Appl Poult Res 11:29–33
Cagle GD, Owen GR, Ridruejo NJ, Wall GM (2001) Compositions for removing human cerumen. Patent: EP1337228
Cai S, Huang Z, Cao Z, Zhang, Zhou M (2008) Inducement and preparation of Stenotrophomonas maltophilia DHHJ keratinase and method for sorting wool by using the same. Patent: CN101265470
Cai SB, Huang ZH, Zhang XQ, Cao ZJ, Zhou MH, Hong F (2011) Identification of a keratinase producing bacterial strain and enzymatic study for its improvement on shrink resistance and tensile strength of wool and polyester blended fabric. Appl Biochem Biotechnol 163(1):112–126
Cai S (2012) Low-toxin low-harm nail polish. Patent: CN102415954
Cardamone JM (2007) Enzyme-mediated crosslinking of wool. Part I: transglutaminase. Text Res J 77:214
Cavello IA, Hours RA, Cavalitto SF (2012) Bioprocessing of hair waste by Paecilomyces lilacinus as a source of a bleach-stable, alkaline and thermostable keratinase with potential application as a laundry detergent additive: Characterization and wash performance analysis. Biotechnol Res Int 369308
Cheng-gang C, Ji-shuang C, Jiong-jiong Q, Yun Y, **ao-dong Z (2008) Purification and characterization of keratinase from a new Bacillus subtilis strain. J Zhejiang Univ Sci B 9(9):713–20
Cheng-gang C, **ao-lu X, **ao-dong Z (2011) Keratin degradation and dehairing application of keratinase from a new Bacillus subtilis strain. IEEE 978-1-4244-5089-3/11/$26.00 ©2011 IEEE
Cortez J, Bonner PLR, Griffin M (2004) Application of transglutaminases in the modification of wool textiles. Enzyme Microb Technol 34:64–72
Coward-Kelly G, Agbogbo FK, Holtzapple MT (2006) Lime treatment of keratinous materials for the generation of highly digestible animal feed: Animal hair. Bioresour Technol 97:1344–1352
Dance A (2008) Prions jump species barrier. Nature. doi:10.1038/news.2008.1080
Ding S, Sun H (2009) Preparation method of cutin dispelling cosmetics and use method. Patent: CN101396328
Encarna P, Elena FK (2011) Tape, in particular, adhesive tape, for the treatment of skin disorders comprising at least one hyperkeratosis inhibitor and/or at least one keratinolytic agent. Patent: WO2011050947
Fakh-fakh-Zauari N, Haddar A, Hmidet N, Farikha F, Moncef N (2010) Application of statistical experimental design for optimization of keratinases production by Bacillus pumilus A1 grown on chicken feather and some biochemical properties. Process Biochem 45:617–626
Freddi G, Mossotti R, Innocenti R (2003) Degumming of silk fabric with several proteases. J Biotechnol 106:101–112
Gradisar H, Friedrich J, Krizaj I, Jerala R (2005) Similarities and specificities of fungal keratinolytic proteases: comparison of keratinases of Paecilomyces marquandii and Doratomyces microsporus to some known proteases. Appl Env Microbiol 71:3420–3426
Gupta R, Ramnani P (2006) Microbial keratinases and their prospective applications: an overview. Appl Microbiol Biotechnol 70:21–33
Gupta R, Sharma R, Beg QK (2012) Revisiting microbial keratinases: next generation proteases for sustainable biotechnology. Crit Rev Biotechnol 30:1–13
Hossain KMG, Juan AR, Tzanov T (2008) Simultaneous protease and transglutaminase treatment for shrink resistance of wool. Biocatal Biotransform 26:405–411
Hui Z, Doi H, Kanouchi H, Matsuura Y, Mohri S, Nonomura Y, Oka T (2004) Alkaline serine protease produced by Streptomyces sp. degrades PrPSc. Biochem Biophys Res Commun 321:45–50
Infante I, Morel MA, Ubalde MC, Martinez-Rosales C, Belvisi S, Castro-Sowninski S (2010) Wool degrading Bacillus isolates: extracellular protease production for microbial processing of fabrics. Wolrd J Microbiol Biotechnol 26:1047–1052
Ismail AMS, Housseiny MM, Abo-Elmagd HI, El-Sayed NH, Habib M (2012) Novel keratinase from Trichoderma harzianum MH-20 exhibiting remarkable dehairing capabilities. Int Biodeterior Biodegrad 70:14–19
Itsune O, Isao M, Keizo H, Naoya I, Mayumi H, Hisami M (2002) Cleaning agent composition. Patent: JP2002256294
Johnson CJ, Bennett JP, Biro SM, Duque-Velasquez JC, Rodriguez CM, Bessen RA, Rocke TE (2011) Degradation of the disease-associated prion protein by a serine protease from lichens. PLoS One 6(5):e19836
Karthikeyan R, Balaji S, Sehgal PK (2007) Industrial applications of keratins—a review. J Sci Ind Res 66(9):710–715
Khardenavis AA, Kapley A, Purohit HJ (2009) Processing of poultry feathers by alkaline keratin hydrolyzing enzyme from Serratia sp. HPC 1383. Waste Manag 29:1409–1415
Konwarh R, Karak N, Rai SK, Mukherjee AK (2009) Polymer-assisted iron oxide magnetic nanoparticle immobilized keratinase. Nanotechnol 20(22):225107
Kornillowicz-Kowalska T, Bohacz J (2011) Biodegradation of keratin waste: theory and practical aspects. Waste Manag 31(8):1689–701
Kumar DJM, Priya P, Balasundari SN, Devi GSDN, Rebecca AIN, Kalaichelvan PT (2012) Production and optimization of feather protein hydrolysate from Bacillus sp. MPTK6 and its antioxidant potential. Middle-East J Sci Res 11(7):900–907
Kumar TP, Raju PN (2013) Transungual drug delivery: a promising route to treat nail disorders. Int J Pharm Sci Rev Res 2(4):22–33
Langveld JPM, Wang JJ, Van de Wiel DFM, Shih JC, Garssen GJ, Bossers A, Shih JCH (2003) Enzymatic degradation of prion protein in brain stem from infected cattle and sheep. J Infect Dis 188:1782–1789
Liu B, Zhang J, **angru-Liao BL, Du G, Chen J (2013) Expression and characterization of extreme alkaline, oxidation-resistant keratinase from Bacillus licheniformis in recombinant Bacillus subtilis WB600 expression system and its application in wool fiber processing. World J Microbiol Biotechnol 29(5):825–832
Lv LX, Sim MH, Li YD, Min J, Feng WH, Guan WJ, Li YQ (2010) Production, characterization and application of a keratinase from Chryseobacterium L99 sp. nov. Process Biochem 45(8):1236–1244
Macedo AJ, Silva WOB, Gava R, Driemeier D, Henriques JAP, Termignoni C (2005) Novel keratinase from Bacillus subtilis S14 exhibiting remarkable dehairing capabilities. Appl Environ Microbiol 71:594–596
Madhavi J, Srilakshmi RMVR, Rao KRSS (2011) Efficient leather dehairing by bacterial thermostable protease. Int J Biosci Biotechnol 3:4
Malhotra GG, Zatz JL (2002) Investigation of nail permeation enhancement by chemical modification using water as a probe. J Pharm Sci 91:312–323
Marcone MF (2005) Characterization of the edible bird’s nest the “Caviar of the East”. Food Res Int 38:1125–1134
Masato Y, Shinji Y, Yasuko M (2011) Thermoprotecting agent for hair. Patent: JP2011046632
Mitsuiki S, Hui Z, Matsumoto D, Sakai M, Moriyama Y, Furukawa K, Kanouchi H, Oka T (2006) Degradation of PrPSc by keratinolytic protease from Nocardiopsis sp TOA-1. Biosci Biotechnol Biochem 70(5):1246–1248
Mohorcic M, Torkar A, Friedrich J, Kristl J, Murdan S (2007) An investigation into keratinolytic enzymes to enhance ungual drug delivery. Int J Pharm 332:196–201
Moore RA, Vorberg I, Priola SA (2005) Species barriers in prion diseases—brief review. Arch Virol Suppl 19:187–202
More SV, Khandelwal HB, Joseph MA, Laxman RS (2013) Enzymatic degumming of silk with microbial proteases. J Nat Fibers 10(2):98–111
Murdan S (2007) Drug delivery to the nail following topical application. Int J Pharm 236:1–26
Nashy EHA, Ahmady AM (2012) Keratinolytic activity of Aspergillus nodulans on dehairing of ovine hide. Egy J Biomed Sci 23:03
Niinimaki A, Niinimaki M, Makinen-Kiljunen S, Hannuksela M (1998) Contact urticaria from protein hydrolysates in hair conditioners. Allergy 53:1075–1082
Noriyuki K, Shoko Y, Etsuji C, Michio K, Shuji Y, Yoshiaki I (2003) Keratinase. Patent: JP2003070461
Odetallah NH, Wang JJ, Garlich JD, Shih JCH (2003) Keratinase in starter diets improves growth of broiler chicks. Poult Sci 82:664–670
Odetallah NH, Wang JJ, Garlich JD, Shih JCH (2005) Versazyme supplementation of broiler diets improves market growth performance. Pout Sci 84:858–864
Okoroma EA, Purchase D, Garelick H, Morris R, Neale MH, Windl O, Abiola OO (2013) Enzymatic formulation capable of degrading scrapie prion under mild digestion conditions. PLoS One 8(7):e68099. doi:10.1371/journal.pone.0068099
Onifade AA, Al-Sane NA, Al-Musallam AA, Al-Zarban S (1998) Potentials for biotechnological applications of keratin-degrading microorganisms and their enzymes for nutritional improvement of feathers and other keratins as livestock feed resources. Bioresour Technol 66:1–11
Paul T, Das A, Mandal A, Jana A, Maity C, Adak A, Halder SK, DasMohapatra PK, Pati BR, Mondal KC (2013) Effective dehairing properties of keratinase from Paenibacillus woosongensis TKB2 obtained under solid state fermentation. Waste Biomass Valor doi:10.1007/s12649-013-9217-z
Prakash P, Jayalakshmi SK, Sreeramulu K (2010) Production of keratinase by free and immobilized cells of Bacillus halodurans strain PPKS-2: partial characterization and its application in feather degradation and dehairing of the goat skin. Appl Biochem Biotechnol 160(7):1909–1920
Rai SK, Mukherjee AK (2011) Optimization of production of an oxidant and detergent-stable alkaline β-keratinase from Brevibacillus sp. strain AS-S10-II: Application of enzyme in laundry detergent formulations and in leather industry. Biochem Eng J 54(1):47–56
Rajput R, Sharma R, Gupta R (2010) Biochemical characterization of a thiol activated, oxidation stable keratinase from Bacillus pumilus KS12. Enzyme Res Enzyme Res 2010:132148
Rajendra VK, Baro A, Kumari A, Dhamecha DL, Lahoti SR, Shelke SD (2012) Transungual drug delivery: an overview. J Appl Pharm Sci 2(1):203–209
Ray A (2012) Protease enzyme—potential industrial scope. Int J Tech 2:1–4
Rutala WA, Weber DJ (2010) Guideline for disinfection and sterilization of prion contaminated medical instruments. Infect Control Hosp Epidemiol 31(2):107–117
Mo SK, Sook KH (2010) Hair cosmetic composition comprising keratinase for protection of hair and repair of hair damage. Patent: KR20110135239
Saunders SE, Bartelt-Hunt SL, Bartz JC (2008) Prions in the environment. Prion 2(4):162–169
Schrooyen PM, Dijkstra PJ, Oberthur RC, Bantjes A, Feijen J (2001) Partially carboxymethylated feather keratins: thermal and mechanical properties of films. J Agric Food Chem 49:221–230
Selvam K, Vishnupriya B (2012) Biochemical and molecular characterization of microbial keratinase and its remarkable applications. Int J Pharm Biological Arch 3(2):267–275
Shen JS, Cavaco-Paulo A, Guubitz GM, Rushforth M, Lenting HBM (2007) Development and industrialisation of enzymatic shrink-resist process based on modified proteases for wool machine washability. Enzyme Microb Technol 40(7):1656–1661
Shigeru F (2012) Skin cosmetic kneaded composition and method for producing same, and method for using skin cosmetic kneaded composition. Patent: US20120305017
Silva CJSM, Zhang Q, Shenb J, Cavaco-Paulo A (2006) Immobilization of proteases with a water soluble–insoluble reversible polymer for treatment of wool. Enzyme Microb Technol 39:634–640
Silva CJSM, Prabaharan M, Gubitz G, Cavaco-Paulo A (2005) Treatment of wool fibres with subtilisin and subtilisin-PEG. Enzyme Microb Technol 36:917–922
Silva CJSM, Sousa F, Gubitz G, Cavaco-Paulo A (2004) Chemical modifications on proteins using glutaraldehyde. Food Technol Biotechnol 42(1):51–56
Sivakumar T, Balamurugan P, Ramasubramanian V (2013) Characterization and applications of keratinase enzyme by Bacillus thuringiensis TS2. Int J Future Biotechnol 2(1):1–8
Sousa F, Jus S, Erbel A, Kokol V, Cavaco-Paulo A, Gubitz GM (2007) A novel metalloprotease from Bacillus cereus for protein fibre processing. Enzyme Microb Technol 40:1772–1781
Spyros T (2003) Use of dual compartment mixing container for enzyme mixtures useful to treat acne. Patent: US6627192
Stark CR, Spencer BE, Shih JCH, Chewing CG, Wang JJ (2009) Evaluation of keratinase stability in pelleted broiler diets. Poultry Sci Ass 30–33
Sumana D, Sudarshan M, Thakur AR, RayChaudhuri S (2013) Degumming of raw silk fabric with help of marine extracellular protease. Am J Biochem Biotechnol 9(1):12–18
Susumu S, Yasuo K, Toshiyuki Y (1998) Skin agent. Patent: JPS63115813
Suzuki Y, Tsujimoto Y, Matsui H, Watanabe K (2006) Decomposition of extremely hard-to-degrade animal proteins by thermophilic bacteria. J Biosci 102:73–81
Taylor DM (2000) Inactivation of transmissible degenerative encephalopathy agents: a review. Vet J 159:10–17
Thomas H, Marlene B, Denise F (2012) Hair treatment products containing one or more surfactant(s) and one or more cationic keratin hydrolysates. Patent: WO2012084336
Tiwary E, Gupta R (2010) Subtilisin-γ-glutamyl transpeptidase: a novel combination as ungual enhancer for prospective topical application. J Pharm Sci 99(12):4866–4873
Tsiroulnikov K, Rezai H, Bonch-Osolovskaya E, Nedkov P, Gousterova A, Cueff V, Godfroy A, Barbier G, Metro F, Chobert JM, Clayette P, Dormont D, Grosclaude J, Haertle T (2004) Hydrolysis of the amyloid prion protein and non-pathogenic meat and bone meal by anaerobic thermophilic prokaryotes and Streptomyces subspecies’. J Agri Food Chem 52:6353–6360
Villa ALV, Aragao MRS, Santos EP, Mazotto AM, Zingali RB, Souza EP, Vermelho AB (2013) Feather keratin hydrolysates obtained from microbial keratinases: effect on hair fiber. BMC Biotechnol 13:15
Wang D, Piao XS, Zeng ZK, Lu T, Zhang Q, Li PF, Xue LF, Kim SW (2011a) Effects of keratinase on performance, nutrient utilization, intestinal morphology, intestinal ecology and inflammatory response of weaned piglets fed diets with different levels of crude protein. J Anim Sci 24(12):1718–1728
Wang HY, Guo YM, Shih JCH (2008) Effects of dietary supplementation of keratinase on growth performance, nitrogen retention and intestinal morphology of broiler chickens fed diets with soybean and cottonseed meals. Anim Feed Sci Technol 140:376–384
Wang JJ, Garlich JD, Shih JCH (2006) Beneficial effects of Versazyme, a keratinase feed additive, on body weight, feed conversion, and breast yield of broiler chickens. J Appl Poult Res 15:544–550
Wang JJ, Swaisgood HC, Shih JCH (2003) Production and characterization of bio-immobilized keratinase in proteolysis and keratinolysis. Enzyme Microb Technol 32:812–819
Wang P, Cui L, Wang Q, Fan X, Zhao X, Wu J (2010) Combined use of mild oxidation and cutinase/lipase pretreatments for enzymatic processing of wool fabrics. Eng Life Sci 10(1):19–25
Wang P, Wang Q, Cui L, Gao M, Fan X (2011b) The combined use of cutinase, keratinase and protease treatments for wool bio-antifelting. Fiber Polym 12(6):760–764
Wang P, Wang Q, Fan X, Cui L, Yuan J, Chen S, Wu J (2009) Effects of cutinase on the enzymatic shrink-resist finishing of wool fabrics. Enzyme Microb Technol 44(5):302–308
Yang Y (2012) Skin-whitening and freckle-dispelling essence and preparation method thereof. Patent: CN102614104
Yin M (2012) Mung-bean shampoo. Patent: CN102451124
Yong PH, U OH, Ha SD, O JM, Gyeong AA (2009) Composition for washing feet. Patent: KR20030079353
Zhang S, Long L, Yin H, **ao Z, Li Q, Zhang S, Tian X, Li C, Chen S, Yin T, Chen Y (2010) Pearl albefaction method mediated by keratinase and combined with redox. Patent: CN100579412
Acknowledgments
Financial assistance from Department of Biotechnology, New Delhi through project number BT/PR012505/PID/06/07/009, University of Delhi, Misc. R&D and DU/DST-Purse is duly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gupta, R., Rajput, R., Sharma, R. et al. Biotechnological applications and prospective market of microbial keratinases. Appl Microbiol Biotechnol 97, 9931–9940 (2013). https://doi.org/10.1007/s00253-013-5292-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-013-5292-0