Abstract
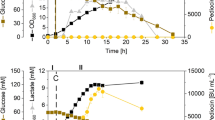
Lactic acid bacteria (LAB) are used widespread in the food industry as traditional starters for various fermented foods. For recombinant protein production, LAB would be superior with view from the food safety demands since most of them are Generally Recognized As Safe organisms. We investigated the two pSIP expression systems, pSIP403 and pSIP409 (Sørvig et al. 2005), to produce a hyper-thermophilic β-glycosidase (CelB) from Pyrococcus furiosus in Lactobacillus plantarum NC8 and Lactobacillus casei as hosts, respectively. Both lactobacilli harboring the pSIP409-celB vector produced active CelB in batch bioreactor cultivations (MRS medium) while the specific CelB activity of the cell free extract was about 44 % higher with L. plantarum (1,590 ± 90 nkat/mgprotein) than with L. casei (1,070 ± 66 nkat/mgprotein) using p-nitrophenyl-β-galactoside (pNPGal) as the substrate. A fed-batch bioreactor cultivation of L. plantarum NC8 pSIP409-celB resulted in a specific CelB activity of 2,500 ± 120 nkat pNPGal/mgprotein after 28 h. A repeated dosage of the inducer spp-IP did not increase the enzyme expression further. As alternative for the cost intensive MRS medium, a basal whey medium with supplements (yeast extract, Tween 80, NH4-citrate) was developed. In bioreactor cultivations using this medium, about 556 ± 29 nkat pNPGal/mgprotein of CelB activity was achieved. It was shown that both LAB were potential expression hosts for recombinant enzyme production. The pSIP expression system can be applied in L. casei.




Similar content being viewed by others
References
Aukrust T, Blom H (1992) Transformation of Lactobacillus strains used in meat and vegetable fermentations. Food Res Int 25:253–261
Ausubel FM (1994) Current Protocols in Molecular Biology, vol 1. John Wiley and Sons, New York
Bernardeau M, Vernoux JP, Henri-Dubernet S, Gueguen M (2008) Safety assessment of dairy microorganisms: the Lactobacillus genus. Int J Food Microbiol 126(3):278–285
Binishofer B, Moll I, Henrich B, Blasi U (2002) Inducible promoter-repressor system from the Lactobacillus casei phage phiFSW. Appl Environ Microbiol 68(8):4132–4135
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Brinques GB, do Carmo Peralba M, Ayub MAZ (2010) Optimization of probiotic and lactic acid production by Lactobacillus plantarum in submerged bioreactor system. J Ind Microbiol Biot 37:205–212
Canchaya C, Claesson MJ, Fitzgerald GF, van Sinderen D, O'Toole PW (2006) Diversity of the genus Lactobacillus revealed by comparative genomics of five species. Microbiology + 152:3185–3196
Cuozzo SA, Sesma F, Palacios JM, de Ruiz Holgado AP, Raya RR (2000) Identification and nucleotide sequence of genes involved in the synthesis of lactocin 705, a two-peptide bacteriocin from Lactobacillus casei CRL 705. FEMS Microbiol Lett 185(2):157–161
De Man JC, Rogosa M, Sharpe ME (1960) A medium for the cultivation of lactobacilli. J Appl Bact 23(1):130–135
de Ruyter PG, Kuipers OP, de Vos WM (1996) Controlled gene expression systems for Lactococcus lactis with the food-grade inducer nisin. Appl Environ Microbiol 62(10):3662–3667
de Vos WM (1999) Gene expression systems for lactic acid bacteria. Curr Opin Microbiol 2(3):289–295
Fischer L, Bromann R, Kengen SW, de Vos WM, Wagner F (1996) Catalytical potency of beta-glucosidase from the extremophile Pyrococcus furiosus in glucoconjugate synthesis. Biotechnology (N Y) 14(1):88–91
Giuliano M, Schiraldi C, Marotta MR, Hugenholtz J, De Rosa M (2004) Expression of Sulfolobus solfataricus alpha-glucosidase in Lactococcus lactis. Appl Microbiol Biotechnol 64(6):829–832
Halbmayr E, Mathiesen G, Nguyen T-H, Maischberger T, Peterbauer CK, Eijsink VGH, Haltrich D (2008) High-level expression of recombinant β-galactosidases in Lactobacillus plantarum and Lactobacillus sakei using a sakacin p-based expression system. J Agr Food Chem 56(12):4710–4719
Kengen SW, Luesink EJ, Stams AJ, Zehnder AJ (1993) Purification and characterization of an extremely thermostable beta-glucosidase from the hyperthermophilic archaeon Pyrococcus furiosus. European journal of biochemistry / FEBS 213(1):305–312
Konings WN, Kok J, Kuipers OP, Poolman B (2000) Lactic acid bacteria: the bugs of the new millennium. Curr Opin Microbiol 3(3):276–282
Kulozik U, Wilde J (1999) Rapid lactic acid production at high cell concentrations in whey ultrafiltrate by Lactobacillus helveticus. Enzyme Microb Tech 24:297–302
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227(5259):680–685
Lebbink JH, Kaper T, Kengen SW, van der Oost J, de Vos WM (2001) beta-Glucosidase CelB from Pyrococcus furiosus: production by Escherichia coli, purification, and in vitro evolution. Method Enzymol 330:364–379
Mathiesen G, Sorvig E, Blatny J, Naterstad K, Axelsson L, Eijsink VG (2004) High-level gene expression in Lactobacillus plantarum using a pheromone-regulated bacteriocin promoter. Lett Appl Microbiol 39(2):137–143
Mayer J, Conrad J, Klaiber I, Lutz-Wahl S, Beifuss U, Fischer L (2004) Enzymatic production and complete nuclear magnetic resonance assignment of the sugar lactulose. J Agr Food Chem 52(23):6983–6990
Mayer J, Kranz B, Fischer L (2010) Continuous production of lactulose by immobilized thermostable beta-glycosidase from Pyrococcus furiosus. J Biotechnol, vol 145:387–493
Mierau I, Kleerebezem M (2005) 10 years of the nisin-controlled gene expression system (NICE) in Lactococcus lactis. Appl Microbiol Biotechnol 68(6):705–717
Peterbauer C, Maischberger T, Haltrich D (2011) Food grade gene expression in lactic acid bacteria. Biotechnol J 6:1147–1161
Renault P (2002) Genetically modified lactic acid bacteria: applications to food or health and risk assessment. Biochimie 84(11):1073–1087
Sawatari Y, Hirano T, Yokota A (2006) Development of food grade media for the preparation of Lactobacillus plantarum starter culture. J Gen Appl Microbiol 52(6):349–356
Schiraldi C, Adduci V, Valli V, Maresca C, Giuliano M, Lamberti M, Carteni M, De Rosa M (2003) High cell density cultivation of probiotics and lactic acid production. Biotechnol Bioeng 82(2):213–222
Sørvig E, Gronqvist S, Naterstad K, Mathiesen G, Eijsink VG, Axelsson L (2003) Construction of vectors for inducible gene expression in Lactobacillus sakei and L plantarum. FEMS Microbiol Lett 229(1):119–126
Sørvig E, Mathiesen G, Naterstad K, Eijsink VG, Axelsson L (2005) High-level, inducible gene expression in Lactobacillus sakei and Lactobacillus plantarum using versatile expression vectors. Microbiology+ 151(Pt 7):2439–2449
Strohmaier W (1998) Lactulose: status of health-related applications. Int Dairy F 9804:262–271
Takala TM, Saris PE, Tynkkynen SS (2003) Food-grade host/vector expression system for Lactobacillus casei based on complementation of plasmid-associated phospho-beta-galactosidase gene lacG. Appl Microbiol Biotechnol 60(5):564–570
Trinetta V, Rollini M, Manzoni M (2008) Development of a low cost culture medium for sakacin a production by L. sakei. Process Biochem 43:1275–1280
Viitanen MI, Vasala A, Neubauer P, Alatossava T (2003) Cheese whey-induced high-cell-density production of recombinant proteins in Escherichia coli. Microb Cell Fact 2(1):2
Wells JM, Mercenier A (2008) Mucosal delivery og therapeutic and prophylactic molecules using lactic acid bacteria. Nat Rev Microbiol 6:349–362
Wong C, Sridhara S, Bardwell JC, Jakob U (2000) Heating greatly speeds Coomassie blue staining and destaining. BioTechniques 28(3):426–428, 430, 432
Acknowledgements
The authors wish to thank Dr. Lars Axelsson for providing the plasmid pSIP403, plasmid pSIP409, and L. plantarum NC8. Further thanks the group of Prof. de Vos and Dr. Kengen, University of Wageningen (NL), for the abandoning of CelB gene. Parts of the research were financed by the German Federal Ministry of Economics and Technology (AiF/FEI Project No. 15801 N) which is greatly acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Böhmer, N., Lutz-Wahl, S. & Fischer, L. Recombinant production of hyperthermostable CelB from Pyrococcus furiosus in Lactobacillus sp.. Appl Microbiol Biotechnol 96, 903–912 (2012). https://doi.org/10.1007/s00253-012-4212-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-012-4212-z