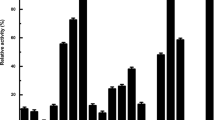
Abstract
Soil metagenome constitutes a reservoir for discovering novel enzymes from the unculturable microbial diversity. From three plant rhizosphere metagenomic libraries comprising a total of 142,900 members of recombinant plasmids, we obtained 14 recombinant fosmids that exhibited lipolytic activity. A selected recombinant plasmid, pFLP-2, which showed maximum lipolytic activity, was further analyzed. DNA sequence analysis of the subclone in pUC119, pELP-2, revealed an open reading frame of 1,191 bp encoding a 397-amino-acid protein. Purified EstD2 exhibited maximum enzymatic activity towards p-nitrophenyl butyrate, indicating that it is an esterase. Purified EstD2 showed optimal activity at 35 °C and at pH 8.0. The K m and K cat values were determined to be 79.4 μM and 120.5/s, respectively. The esterase exhibited an increase in enzymatic activity in the presence of 15% butanol and 15% methanol. Phylogenetic analysis revealed that the lipolytic protein EstD2 may be a member of a novel family of lipolytic enzymes. Several hypothetical protein homologs of EstD2 were found in the database. A hypothetical protein from Phenylobacterium zucineum HLK1, a close homolog of EstD2, displayed lipolytic activity when the corresponding gene was expressed in Escherichia coli. Our results suggest that the other hypothetical protein homologs of EstD2 might also be members of this novel family.




Similar content being viewed by others
References
Amann RI, Ludwig W, Schleifer KH (1995) Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 59:143–169
Arpigny JL, Jaeger KE (1999) Bacterial lipolytic enzymes: classification and properties. Biochem J 343:177–183
Atkinson D, Watson CA (2000) The beneficial rhizosphere: a dynamic entity. Appl Soil Ecol 15:99–104
Beloqui A, Pita M, Polaina J, Martíez-Arias A, Golyshina OV, Zumárraga M, Yakimov MM, García-Arellano H, Alcalde M, Fernández VM, Elborough K, Andreu JM, Ballesteros A, Plou FJ, Timmis KN, Ferrer M, Golyshin PN (2006) Novel polyphenol oxidase mined from a metagenome expression library of bovine rumen: biochemical properties, structural analysis, and phylogenetic relationships. J Biol Chem 281:22933–22942
Chung EJ, Lim HK, Kim JC, Choi GJ, Park EJ, Lee MH, Chung YR, Lee SW (2008) Forest soil metagenome gene cluster involved in antifungal activity expression in Escherichia coli. Appl Environ Microbiol 74:723–730
Cottrell MT, Moore JA, Kirchman DL (1999) Chitinases from uncultured marine microorganisms. Appl Environ Microbiol 65:2553–2557
Elend C, Schmeisser C, Leggewie C, Babiak P, Carballeria JD, Steele HL, Reymond JL, Jaeger KE, Streit WR (2006) Isolation and biochemical characterization of two novel metagenome-derived esterases. Appl Environ Microbiol 72:3637–3645
Ferrer M, Golyshina OV, Chernikova TN, Khachane AN, Martins dos Santos VAP, Yakimov MM, Timmis KN, Golyshin PN (2005) Microbial enzymes mined from the Urania deep-sea hypersaline anoxic basin. Chem Biol 12:895–904
Handelsman J (2004) Metagenomics: application of genomics to uncultured microorganisms. Microbiol Mol Biol Rev 68:669–685
Harrison CJ, Langdale JA (2006) A step by step guide to phylogeny reconstruction. Plant J 45:561–572
Hasan F, Shah AA, Hameed A (2006) Industrial applications of microbial lipases. Enzyme Microb Technol 39:235–251
Henne A, Schmitz RA, Bömeke M, Gottschalk G, Daniel R (2000) Screening of environmental DNA libraries for the presence of genes conferring lipolytic activity of Escherichia coli. Appl Environ Microbiol 66:3113–3116
Hugenholtz P, Pace NR (1996) Identifying microbial diversity in the natural environment: a molecular phylogenetic approach. Trends Biotechnol 14:190–197
Jaeger KE, Eggert T (2002) Lipases for biotechnology. Curr Opin Biotechnol 13:390–397
Kim EY, Oh KH, Lee MH, Kang CH, Oh TK, Yoon JH (2009a) Novel cold-adapted alkaline lipase from intertidal flat metagenome and proposal of a new family of bacterial lipase. Appl Environ Microbiol 75:257–260
Kim JS, Lim HK, Lee MH, Park JH, Hwang EC, Moon BJ, Lee SW (2009b) Production of porphyrin intermediates in Escherichia coli carrying soil metagenomic genes. FEMS Microbiol Lett 295:42–49
Lee DG, Jeon JH, Jang MK, Kim NY, Lee JH, Lee JH, Kim SJ, Kim GD, Lee SH (2007) Screening and characterization of a novel fibrinolytic metalloprotease from a metagenomic library. Biotechnol Lett 29:465–472
Lee MH, Lee CH, Oh TK, Song JK, Yoon JH (2006) Isolation and characterization of a novel lipase from a metagenomic library of tidal flat sediments: evidence for a new family of bacterial lipases. Appl Environ Microbiol 72:7406–7409
Lee SW, Won K, Lim HK, Kim JC, Choi GJ, Cho KY (2004) Screening for novel lipolytic enzymes from uncultured soil microorganisms. Appl Microbiol Biotechnol 65:720–726
Lim HK, Chung EJ, Kim JC, Choi GJ, Jang KS, Chung YR, Cho KY, Lee SW (2005) Characterization of a forest soil metagenome clone that confers indirubin and indigo production on Escherichia coli. Appl Environ Microbiol 71:7768–7777
Luo Y, Xu X, Ding Z, Liu Z, Zhang B, Yan Z, Sun J, Hu S, Hu X (2008) Complete genome of Phenylobacterium zucineum—a novel facultative intracellular bacterium isolated from human erythroleukemia cell line K562. BMC Genomics 9:386
Nierman WC, Feldblyum TV, Laub MT, Paulsen IT, Nelson KE, Eisen J, Heidelberg JF, Alley MRK, Ohta N, Maddock JR, Potocka I, Nelson WC, Newton A, Stephens C, Phadke ND, Ely B, DeBoy RT, Dodson RJ, Durkin SA, Gwinn ML, Haft DH, Kolonay JF, Smit J, Craven MB, Khouri H, Shetty J, Berry K, Utterback T, Tran K, Wolf A, Vamathevan J, Ermolaeva M, White O, Salzberg SL, Venter CJ, Shapiro L, Fraser CM (2001) Complete genome sequence of Caulobacter crescentus. Proc Natl Acad Sci U S A 98:4136–4141
Park SY, Kim JT, Kang SG, Woo JH, Lee JH, Choi HT, Kim SJ (2007) A new esterase showing similarity to putative dienelactone hydrolase from a strict marine bacterium, Vibrio sp. GMD509. Appl Microbiol Biotechnol 77:107–115
Rees HC, Grant S, Jones B, Grant WD, Heaphy S (2003) Detecting cellulase and esterase enzyme activities encoded by novel genes present in environmental DNA libraries. Extremophiles 7:415–421
Robertson DE, Chaplin JA, DeSantis G, Podar M, Madden M, Chi E, Richardson T, Milan A, Miller M, Weiner DP, Wong K, McQuaid J, Farwell B, Preston LA, Tan X, Snead MA, Keller M, Mathur E, Kretz PL, Burk MJ, Short JM (2004) Exploring nitrilase sequence space for enantioselective catalysis. Appl Environ Microbiol 70:2429–2436
Rondon MR, August PR, Bettermann AD, Brady SF, Grossman TH, Liles MR, Loiacono KA, Lynch BA, MacNeil IA, Minor C, Tiong CL, Gilman M, Osburne MS, Clardy J, Handelsman J, Goodman RM (2000) Cloning the soil metagenome: a strategy for accessing the genetic and functional diversity of uncultured microorganisms. Appl Environ Microbiol 66:2541–2547
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning. A laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, New York
Sandkvist M (2001) Type II secretion and pathogenesis. Infect Immun 69:3523–3535
Shah J (2005) Lipids, lipases, and lipid-modifying enzymes in plant disease resistance. Annu Rev Phytopathol 43:229–260
Simon C, Daniel R (2009) Achievements and new knowledge unraveled by metagenomic approaches. Appl Microbiol Biotechnol 85:265–276
Swofford DL (2003) PAUP*: Phylogenetic Analysis Using Parsimony (* and Other Methods), Version 4.0b 10. Sunderland, MA: Sinauer Associates
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Hoggins DG (1997) The CLUSTAL-X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25:4876–4882
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Wang X (2004) Lipid signaling. Curr Opin Plant Biol 7:329–336
Yun J, Kang S, Park S, Yoon H, Kim MJ, Heu S, Ryu S (2004) Characterization of a novel amylolytic enzyme encoded by a gene from a soil-derived metagenomic library. Appl Environ Microbiol 70:7229–7235
Zhou J, Bruns MA, Tiedje JM (1996) DNA recovery from soils of diverse composition. Appl Environ Microbiol 62:316–322
Acknowledgments
This research was supported by a grant (MG 08-0104-1-0) from the Microbial Genomics and Application Center of the 21st Century Frontier Research Program funded by the Ministry of Education, Science and Technology of the Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Additional information
Myung Hwan Lee and Kyung Sik Hong contributed equally to this study.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Fig. S1
SDS-PAGE gel showing the expressed EstD2 protein produced in E. coli and purified EstD2. a Expression of EstD2 in E. coli BL21(DE3)/pLysS carrying pELP28. To induce his-tag fusion protein in E. coli, 1 mM of IPTG was added to the bacterial culture from time to time. Lanes: S molecular weight standards with sizes written on the left; 1 before IPTG addition; 2, 4, 6, 8 uninduced culture (1, 2, 3, and 4 h without IPTG addition); 3, 5, 7, 9 induced culture (1, 2, 3, and 4 h after IPTG addition). Arrow indicates the expressed protein. b Purified EstD2 protein by affinity purification. Lanes: S molecular weight standards with sizes noted on the left; 1 purified protein (arrow). (PPT 255 kb)
Rights and permissions
About this article
Cite this article
Lee, M.H., Hong, K.S., Malhotra, S. et al. A new esterase EstD2 isolated from plant rhizosphere soil metagenome. Appl Microbiol Biotechnol 88, 1125–1134 (2010). https://doi.org/10.1007/s00253-010-2729-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00253-010-2729-6