Abstract
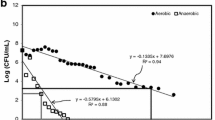
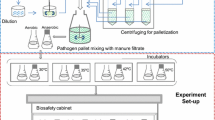
This study was conducted to identify an indicator organism(s) in evaluating the pathogen-reducing capacity of biogas plants. Fresh cow manure containing 104 to 105 colony forming unit (CFU) per milliliter of Escherichia coli and Enterococcus faecalis along with an inoculated Clostridium perfringens strain were exposed to 37°C for 15 days, 55°C for 48 h, and 70°C for 24 h. C. perfringens was the most heat-resistant organism followed by E. faecalis, while E. coli was the most heat-sensitive organism. E. coli was reduced below detection limit at all temperatures with log10 reductions of 4.94 (10 s), 4.37 (40 min), and 2.6 (5 days) at 70°C, 55°C, and 37°C, respectively. Maximum log10 reductions for E. faecalis were 1.77 at 70°C (1 day), 1.7 at 55°C (2 days) and 3.13 at 37°C (15 days). For C. perfringens, maximum log10 reduction at 37°C was 1.35 log10 units (15 days) compared to less than 1 unit at 55 and 70°C. Modeling results showed that E. faecalis and C. perfringens had higher amount of heat-resistant fraction than E. coli. Thus, E. faecalis and C. perfringens can be used as indicator organisms to evaluate pathogen-reducing capacity in biogas plants at high temperatures of 55°C and 70°C while at 37°C E. coli could also be included as indicator organism.


Similar content being viewed by others
References
Aitken MD, Sobsey MD, Van Abel NA, Blauth KE, Crunk PL, Melsch ME, Nichols CC (2005) Inactivation of Escherichia coli O157:H7 during thermophilic anaerobic digestion of manure from dairy cattle. Proc 10th World Congress—Anaerobic Digestion 2:377–382
Albihn A, Eriksson E, Wallen C, Aspán A (2002) Verotoxinogenic E. coli (VTEC) O157:H7—A nation-wide Swedish survey of bovine faeces. Acta Vet Scand 44:43–52
Animal by-Products Regulation (2002) Impacts on composting and Anaerobic Digestion. The European Parliament and the Council of the European Union, Brussels, Regulation (EC) No 1774/2002
APHA (1998) In: Lenore SC, Greenberg AE, Eaton AD (eds) Standard Methods for the Examination of Water and Wastewater, 20th edn. American Public Health Association, Washington DC
Bendixen HJ (1994) Safeguards against pathogens in Danish Biogas Plants. Wat Sci Tech 30:171–180
Bendixen HJ (1999) Hygienic safety—results of scientific investigations in Denmark Sanitation requirements in Danish Biogas Plants. In: Bohn R, Wellinger A (eds) Hygienic and Environmental Aspects of Anaerobic Digestion: Legislation and Experiences in Europe, Stuttgart-Hohenheim. Universität Hohenheim, Stuttgart, pp 27–47
Bendixen HJ, Ammendrup S (1992) Safeguards against pathogens in biogas plants. Practical measure to prevent dissemination of pathogens and requirements for sanitation. Danish Vet. Service, Copenhagen, 32 pp
Bicudo JR, Goyal SM, Zhu J (2000) Animal production, manure management and pathogens: A review. In: Animal, Agricultural and Food Processing Wastes. Moore JA (ed) Proc of the 8th International Symposium, 09–11.10.2000, Des Moines, Iowa, USA. American Society of Agricultural Engineers, St. Joseph, Michigan, USA. pp. 507–521
Bitton G (2005) Microbial indicators of fecal contamination: Application to microbial source tracking, report submitted to Florida Stormwater Association
Byrne B, Dunne G, Bolton DJ (2006) Thermal inactivation of Bacillus cereus and Clostridium perfringens vegetative cells and spores in port luncheon roll. Food Microbiol 23:803–808
Carrington EG (2001) Evaluation of sludge treatments for pathogen reduction-Final report. Study contract no. B4–3040/2001/322179/MAR/A2, For the European Commission directorate-General Environment. ISBN 92–894–1734-X. Available at: http://europa.eu.int/comm/enivronment/waste/sludge/sludge_eval.pdf. (visited on July 2006)
Colleran E (2000) Hygienic and sanitation requirements in biogas plants treating animal manure or mixtures of manure and other organic waste. In: Ortenblad H (Ed.) Anaerobic Digestion: Making Energy and Solving Modern Waste Problem 77–86
Demeyer DJ, Henderickx HK (1967) The effects of unsaturated fatty acids on methane production in vitro by mixed rumen bacteria. Biochem Biophys Acta 137:484–497
Espensen B (1996) Praktiske forsög med smitstofreducerende bahandling af husholdingaffald. Dansk Vet Tidsskr 79(14):615–622, (in Danish)
Gibbs RA, Hu CJ, Ho GE, Phillips PA, Unkovich I (1995) Pathogen die-off in stored wastewater sludge. Wat Sci Tech 31:93–95
Harmon SM, Kautter DA, Peeler JT (1997a) Comparison of media for the enumeration of Clostridium perfringens. Appl Microbiol 21(5):922–927
Harmon SM, Kautter DA, Peeler JT (1997b) Improved medium for enumeration of Clostridium perfringens. Appl Microbiol 22(4):688–692
Hartung M, Gerigk K (1991) Yersinia in effluents from the food-processing industry. Rev Sci Tech 10:799–811
Himathongkham S, Bahari S, Riemann H, Cliver D (1999) Survival of Escherichia coli O157:H7 and Salmonella typhimurium in cow manure and cow manure slurry. FEMS Microbiol Lett 178:251–257
ISO (1997) Water Quality—Detection and Enumeration of Escherichia coli and Coliform Bacteria—Part 1: Membrane Filtration Method. International Organization for Standardization, Geneva, ISO/DIS 9308–1
Labbe RG (2001) Clostridium perfringens. In: Downs FP, Ito K (eds) Compendium of Methods for the Microbiological Examination of Foods. American Public Health Association, Washington, DC, pp 325–330
Langeland G, Paulsrud B (1985) Aerobic thermophilic stabilisation. In: Strauch D, Havelaar A, Hermite P (eds) Inactivation of microorganims in sewage sludge by stabilisation process. Elsevier, Amsterdam, pp 38–47
Larsen HE, Munch B, Schlundt J (1994) Use of indicators for monitoring the reduction of pathogens in animal waste treated in biogas plants. Zentralbl Hyg Umveltmed 195:544–555
Lide RD (1997) Handbook of Chemistry and Physics, 77th edn. CRC, Boca Raton
Logue CM, Sheridan JJ, McDowell DA, Blair IS, Hegarty T (2000) The effect of temperature and selective agents on the growth of Yerwina enterocolitica serotype O:3 in pure culture. J Appl Microbiol 88:1001–1008
Mitscherlich E, Marth EH (1984) Microbial Survival in the Environment. Springer, Berlin
Olsen JE, Larsen HE (1987) Bacterial decimation times in anaerobic digestions of animal slurries. Biol Wastes 21:153–168
Oropeza MR, Cabirol N, Ortega S, Ortiz LPC, Noyola A (2001) Removal of fecal indicator organisms and parasites (fecal coliforms and helminth eggs) from municipal biologic sludge by anaerobic mesophilic and thermophilic digestion. Wat Sci Tech 44:97–101
Park GW, Diez-gonzalez F (2003) Utilization of carbonate and ammonia-based treatments to eliminate Escherichia coli O157:H7 and Salmonella Typhimurium DT104 from cattle manure. J Appl Microbiol 94(4):675–685
Park S, Worobo RW, Durst RA (1999) Escherichia coli O157:H7 as an emerging food borne pathogen. A literature review. Critical Rev Food Sci Nut 39:481–502
Plym-Forshell L (1995) Survival of salmonellas and Ascaris suum eggs in a thermophilic biogas plant. Acta vet Scandina 36:79–85
Salsali HR, Parker WJ, Sattar SA (2005) The effect of volatile fatty acids on the inactivation of Clostridium perfringens in advanced anaerobic digestion. Proc. 10th World Congress—Anaerobic digestion 2:486–490
Scanlan P, Santha H, Fronek S (2005) Advanced anaerobic digestion for Class A pathogen removal. Proc 10th World Congress—Anaerobic Digestion 2:491–497
Sinton LW, Donnison AM, Hastie CM (1993) Faecal streptococci as faecal pollution indicators: a review. Part I: Taxonomy and enumeration. N Z J Mar Freshw Res 27:101–115
Slanetz LW, Bartley CH (1957) Numbers of enterococci in water, sewage, and faeces determined by the membrane filter technique with an improved medium. J Bact 74:591–595
Sorensen AH, Winther-Nielsen M, Ahring BK (1991) Kinetics of lactate, acetate and propionate in unadapted and lactate-adapted thermophilic, anaerobic sewage sludge: the influence of sludge adaptation for start-up of thermophilic UASB-reactors. Appl Microbiol Biotechnol 34:823–827
Wery N, Pourcher A-M, Stan V, Delgenes J-P, Picard-Bonnaud F, Godon J-J (2006) Survival of Listeria monocytogenes and Enterococcus faecium in sludge evaluated by real-time PCR and culture methods. Lett Appl Microbiol 43(2):131–136
Zhai Q, Coyne MS, Barnhisel RI (1995) Mortality rates of fecal bacteria in subsoil amended with poultry manure. Bioresour Technol 54:165–169
Van Immerseel F, De Buck J, Pasmans F, Huyghebaert G, Haesebrouck F, Ducatelle R (2004) Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol 33(6):537–549, Review
Acknowledgements
The authors thank Dr. Dimitar Karakashev, Hector Garcia, A. Dilokpimol, V. Siriwongrungson, and Dawei Liu from the Technical University of Denmark and I. B. Jensen from Statens Serum Institute, Denmark for their technical support to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Watcharasukarn, M., Kaparaju, P., Steyer, JP. et al. Screening Escherichia coli, Enterococcus faecalis, and Clostridium perfringens as Indicator Organisms in Evaluating Pathogen-Reducing Capacity in Biogas Plants. Microb Ecol 58, 221–230 (2009). https://doi.org/10.1007/s00248-009-9497-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-009-9497-9