Abstract
Microorganisms differ in their effectiveness in uptake and selection of substances that they bring in from the environment. They also differ in how they balance the allocation of nutrients for immediate and for delayed use. Moreover, they may not take up resources as fast as they seemingly could, and they may extrude derivatives of substances just pumped in. A good deal of these apparent choices must reside in the uptake systems and the linkage of these with the cell’s intermediate metabolism. An important feature is that a resource may vary in concentration from time to time, nutrient to nutrient, and habitat to habitat. This variation must have been critical to the evolution of regulatory processes. Some possibilities for the combined uptake and consumption are considered for substrates serving the same (homologous) and different (heterologous) roles for the bacterium. From the membrane transport processes diagrammed in Fig. 1c and Fig. 2 and corresponding computer program given in Appendix A, the combined effect of uptake processes and cell growth can be studied. The model can be modified for various alternate models to study the possible control of cellular uptake and metabolism for the range of ecological roles of the bacterium.

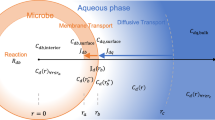
Models for bacterial growth. (a) The Monod model. Monod postulated, analogously to Michaelis–Menten enzyme kinetics, that a reversible complex is formed between the substrate, S, and the bacterium, B. This complex is resolves to recover the original catalyst, i.e., the bacterium, and the product, which is a second bacterium. (b) The Best model. The external substrate, SE, diffuses reversibly through the cell membrane. When internal to the cell, where it is designated as SI, it reacts with a cell enzyme, E. This is the first step in its metabolism. The complex, in an irreversible reaction, regenerates the enzyme, produces a product, P, that then is used variously to supports different aspects of cell growth. (c) Linkage model. This model links uptake with consumption. A pathway for facilitated diffusion acts to bring the substrate into the cell via a membrane-bound carrier, T. All stages (binding, dissociating, traversing the membrane) are reversible. The substrate, on dissociation, reacts internally with the first enzyme in the intermediary metabolism pathway according to the usual kind of enzyme mechanism with a Km and a V′max. Three regulatory mechanisms are shown: (i) by combination with a phosphate group by a two-component system; (ii) by overflow metabolism as the internal substrate is ejected from the cell in a transformed state, X; (iii) by storage within the cell as compound Y.

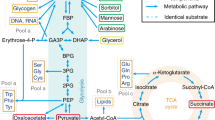
Metabolic scheme for the linkage of uptake of two growth substrates. The transport system for both substrates was modeled from an earlier review [30]. It has been outlined in Fig. 1c. The symbols S, T, E, I stand for substrate, transporter, external, and internal substrate. These symbols have an A or B added to them depending on which transport system is involved. Rate constants have been designated, for example, by K13B instead of K13B. Also KMB is used instead of KMB and VmaxB instead of VmaxB, to designate Michaelis–Menten constants and maximum velocities. In this scheme there are four forms of the free substrates and four forms of the transporter for each substrate. The 12 kinetic rate constants for each substrate should be considered the equivalents of the rate constants in chemical kinetics or in enzymology. The internal form of each kind of substrate, SIA and SIB, feeds into central metabolism at a maximum rate dependent on EA*K13A and EB*K13B when SIA or SIB are large relative to the binding constants, KMA or KMB (i.e., at saturation condition). The internal substrate may also be extruded in another form, XA and XB, or stored as a reserve material inside the cell, YA and YB. These phenomenological constants correspond to different kinetic forms for homologous and heterologous substrates as described in the text. The open-headed arrows present a possible control of the internal pools on that function by regulating TIA and TIB.



Similar content being viewed by others
References
AR Archibald (1976) ArticleTitleCell wall assembly in Bacillus subtilis: development of bacteriophage binding properties as a result of incorporation of teichoic acid J Bacteriol 127 956–960 Occurrence Handle821923
FB Bader (1982) Kinetics of double-substrate limited growth MJ Bazin (Eds) Microbial Population Dynamics CRC Press Boca Raton, Fl 2–32
JB Best (1955) ArticleTitleThe inference of intracellular properties from observed kinetic data J Cell Comp Physiol 46 1–27 Occurrence Handle10.1002/jcp.1030460102
FF Blackman (1905) ArticleTitleOptima and limiting growth Ann Bot 19 281–295
SE Bresler MI Mosevitsky LG Vyacheslavov (1973) ArticleTitleMutations as possible replication errors in bacteria growing under conditions of thymine deficiency Mut Res 19 281–293
GE Briggs JBS Haldane (1925) ArticleTitleA note on the kinetics of enzyme action Biochem J 19 338–339
DK Button F Schut P Quang R Martin BR Robertson (1993) ArticleTitleViability and isolation of marine bacteria by dilution culture: theory procedures and initial result Appl Environ Microbiol 59 881–891
GN Cohen J Monod (1957) ArticleTitleBacterial permeases Bacteriol Rev 21 164–194
SS Cohen (1971) ArticleTitleOn the nature of thymineless death Ann NY Acad Sci 186 292–301
JN Dabes RK Finn CR Wilke (1973) ArticleTitleEquations of substrate-limited growth: the case of Blackman kinetics Biotech Bioeng 15 1159–1177 Occurrence Handle10.1002/bit.260150613
T Egli (1991) ArticleTitleOn multiple-nutrient-limited growth of microorganism with special reference to dual limitation by carbon and nitrogen substrates Antonie van Leeuwenhoek 60 225–234 Occurrence Handle10.1007/BF00430367 Occurrence Handle1687236
T Egli (1995) ArticleTitleThe ecological and physiological significance of the growth of heterotrophic microorganism with mixtures of substrates Adv Microb Ecol 8 305–386
T Egli U Lendenmann M Snozzi (1993) ArticleTitleKinetics of microbial growth with mixtures of carbon sources Antonie van Leeuwenhoek 63 289–298 Occurrence Handle10.1007/BF00871224 Occurrence Handle8279825
DC Ellwood DW Tempest (1967) ArticleTitleTeichoic acid or teichuronic acid in the walls of Bacillus subtilis var. niger grown in a chemostat Biochem J 104 69
T Ferenci (1996) ArticleTitleAdaptation to life at micromolar nutrient levels: the regulation of Escherichia coli glucose transport by endoinduction and cAMP FEMS Microbiol Rev 18 301–316 Occurrence Handle10.1016/0168-6445(96)00019-8 Occurrence Handle8703508
SD Grazer-Lampert T Egli G Hamer (1986) ArticleTitleGrowth of Hyphomicrobium ZV620 in the chemostat: regulation of the NH4+-assimilating enzymes and cellular composition J Gen Microbiol 132 3337–3347
W Harder L Dijkhuisen (1982) ArticleTitleStrategies of mixed substrate utilization in microorganism Phil Trans R Soc Lond B 297 459–480
W Harder L Dijkhuisen (1983) ArticleTitlePhysiological responses to nutrient limitation Ann Rev Microbiol 37 1–23 Occurrence Handle10.1146/annurev.mi.37.100183.000245
MJ Harte FC Webb (1967) ArticleTitleUtilization of mixed sugars in continuous fermentations II Biotechnol Bioeng 9 205–221 Occurrence Handle10.1002/bit.260090207
JZ Hearon (1952) ArticleTitleRate behavior of metabolic systems Physiol Rev 32 499–522 Occurrence Handle13003538
E Hegewald WA Knorre (1978) ArticleTitleKinetics of growth and substrate consumption of Escherichia coli ML 30 on two carbon sources Z Allg Mikrobiol 18 415–426 Occurrence Handle362739
H Kacser JA Burns (1973) ArticleTitleThe control of flux Symp Soc Exper Biol 27 65–104
DB Kell HV Westerhoff (1986) ArticleTitleMetabolic control theory: its role in microbiology and biotechnology FEMS Microbiol Rev 39 305–320 Occurrence Handle10.1016/0378-1097(86)90020-0
AL Koch (1967) ArticleTitleKinetics of permease catalyzed transport J Theor Biol 14 103–130 Occurrence Handle10.1016/0022-5193(67)90109-9 Occurrence Handle5342475
AL Koch (1971) ArticleTitleThe adaptive responses of Escherichia coli to a feast and famine existence Adv Microb Physiol 6 147–217 Occurrence Handle4950180
AL Koch (1972) ArticleTitleDeviations from hyperbolic dependency of transport processes J Theor Biol 36 23–40 Occurrence Handle10.1016/0022-5193(72)90174-9 Occurrence Handle5070903
AL Koch (1982) Diffusion limit and bacterial growth V Krumphanzl B Sikyta Z Vanek (Eds) Overproduction of Microbial Products Academic Press London 571–580
AL Koch (1982) ArticleTitleMultistep kinetics: choice of models for growth of bacteria J Theor Biol 98 401–417 Occurrence Handle10.1016/0022-5193(82)90127-8 Occurrence Handle6757586
AL Koch (1985) The macroeconomics of bacterial growth MM Fletcher GD Floodgate (Eds) Bacteria in Their Natural Evironment Soc Gen Microbiol London 1–42
AL Koch (1996) ArticleTitleWhat size should a bacterium be? A question of scale Ann Rev Microbiol 50 317–348 Occurrence Handle10.1146/annurev.micro.50.1.317
AL Koch (1997) The Monod model, its alternatives AL Koch JA Robinson GA Milliken (Eds) Mathematical Models in Microbial Ecology Chapman Hall New York 62–93
AL Koch (1997) ArticleTitleMicrobial physiology, ecology of slow growth Microbiol Mol Biol Rev 61 305–318 Occurrence Handle9293184
AL Koch (2001) Bacterial Growth and Form EditionNumber2 Kluwer Dordrecht, The Netherlands
Koch, AL (2002) Oligotrophy. Bacterial oligotrophism. Encyclo Environ Microbiol 2267–2277
AL Koch (2001) ArticleTitleOligotrophs versus copiotrophs BioEssays 23 657–661 Occurrence Handle10.1002/bies.1091 Occurrence Handle11462219
AL Koch R Coffman (1970) ArticleTitleDiffusion permeation or enzyme limitation: a probe for the kinetics of enzyme induction Biotechnol Bioeng 11 651–677 Occurrence Handle10.1002/bit.260120503
AL Koch ML Higgins RJ Doyle (1981) ArticleTitleSurface tension–like forces determine bacterial shapes: Streptococcus faecium J Gen Microbiol 123 151–161 Occurrence Handle7320694
AL Koch BR Robertson DK Button (1996) ArticleTitleDeduction of the cell volume, mass from forward scatter intensity analyzed by flow cytometry J Microbiol Methods 27 49–61 Occurrence Handle10.1016/0167-7012(96)00928-1
K Kovarova-Kovar T Egli (1998) ArticleTitleGrowth kinetics of suspended microbial cells: from single-substrate-controlled growth to mixed-substrate kinetics Microbiol Mol Biol Rev 62 646–66 Occurrence Handle9729604
U Lendenmann T Egli (1995) ArticleTitleIs Escherichia coli growing in glucose-limited chemostat culture able to utilize other sugars without lag Microbiology 141 71–78 Occurrence Handle7894722
U Lendenmann M Snozzi T Egli (1996) ArticleTitleKinetics of simultaneous utilization of sugar mixtures by Escherichia coli in continuous culture Appl Environ Microbiol 62 1493–1499 Occurrence Handle8633848
I Malek (Eds) (1958) Continuous Cultivation of Microorganisms: Symposium Czech Acad Sci Prague, Czechoslovakia
T Merad AR Archibald IC Hancock CR Harwood JA Hobot (1989) ArticleTitleCell wall assembly in Bacillus subtilis: visualisation of old, new wall material by electron microscopic examination of samples stained selectively for teichoic acid, and teichuronic acid J Gen Microbiol 35 645–655
HLT Mobley AL Koch RJ Doyle UN Streips (1984) ArticleTitleInsertion, fate of cell wall in Bacillus subtilis J Bacteriol 158 169–179 Occurrence Handle6232259
J Monod (1942) Recherches sur la croissance des cultures bactériennes Hermann Cie Paris
J Monod (1950) ArticleTitleLa technique de culture continue: théorie et applications Ann Inst Pasteur 79 390–410
A Narang (1998) ArticleTitleThe steady states of microbial growth on mixtures of substitutable substrates in a chemostat J Theor Biol 190 24–261 Occurrence Handle10.1006/jtbi.1997.0552
A Narang AE Konopka D Ramkrishna (1997) ArticleTitleThe dynamics of microbial growth on mixtures of substrates in a batch reactor J Theor Biol 184 301–317 Occurrence Handle10.1006/jtbi.1996.0275
OM Neijssel DW Tempest (1976) ArticleTitleThe role of energy-spilling reactions in the growth of Klebsiella aerogenes NCTC 418 in aerobic chemostat culture Arch Microbiol 110 305–311 Occurrence Handle10.1007/BF00690243 Occurrence Handle1015953
A Novick L Szilard (1950) ArticleTitleDescription of the chemostat Science 112 715–716 Occurrence Handle14787503
A Novick L Szilard (1951) ArticleTitleGenetic mechanisms in bacteria, bacterial viruses: I Cold Spr Harb Symp Quant Biol 16 337–342
A Novick L Szilard (1951) ArticleTitleExperiments with the chemostat on spontaneous mutations Proc Natl Acad Sci USA 36 708–719
JS Poindexter (1981) ArticleTitleOligotrophy: fast, famine existence Adv Microb Ecol 5 63–90
EO Powell (1967) The growth rate of micro-organisms as a function of substrate concentration EO Powell CGT Evans RE Strange DW Tempest (Eds) Microbial Physiology. Continuous Culture HMSO London 34–56
EO Powell CGT Evans RE Strange DW Tempest (Eds) (1967) Microbial Physiology, Continuous Culture HMSO London
R Ramakrishna D Ramkrishna AE Konopka (1996) ArticleTitleCybernetic modeling of growth in mixed substitutable substrate environments: preferential, simultaneous utilization Biotechnol Bioeng 52 141–151 Occurrence Handle10.1002/(SICI)1097-0290(19961005)52:1<141::AID-BIT14>3.0.CO;2-R
R Ramakrishna D Ramkrishna AE Konopka (1997) ArticleTitleMicrobial growth on substitutable substrates: characterizing the consumer–resource relationship Biotechnol Bioeng 54 77–90 Occurrence Handle10.1002/(SICI)1097-0290(19970405)54:1<77::AID-BIT9>3.0.CO;2-V
M Rutgers PA Balk K Dam Particlevan (1990) ArticleTitleQuantification of multiple substrate–controlled growth: simultaneous ammonium, glucose limitation in chemostat cultures of Klebsiella pneumoniae Arch Microbiol 153 478–484 Occurrence Handle10.1007/BF00248430 Occurrence Handle2187428
F Schut EJ Vries Particlede JC Gottschal BR Robertson W Harder RA Prins DB Button (1993) ArticleTitleIsolation of typical marine bacteria by dilution culture: growth maintenance, characteristics of isolates under laboratory conditions Appl Environ Microbiol 59 2150–2180
TA Shehata AG Marr (1971) ArticleTitleEffect of nutrient concentration on the growth of Escherichia coli J Bacteriol 107 210–215 Occurrence Handle4935320
RS Silver RI Mateles (1969) ArticleTitleControl of mixed-substrate utilization in continuous culture of Escherichia coli J Bacteriol 97 535–543 Occurrence Handle4886282
DW Tempest JW Dicks JR Hunter (1966) ArticleTitleThe interrelationship between potassium, magnesium and phosphorus in potassium-limited chemostat culture J Gen Microbiol 45 135–146
DW Tempest JR Hunter J Sykes (1965) ArticleTitleMagnesium-limited growth of Aerobacter aerogenes in a chemostat J Gen Microbiol 39 355–366 Occurrence Handle5864530
DW Tempest OM Neijssel (1992) ArticleTitlePhysiological and energetic aspects of bacterial overproduction FEMS Microbiol Lett 100 169–176 Occurrence Handle10.1016/0378-1097(92)90205-3
BG Turner D Ramkrishna NB Jansen (1988) ArticleTitleCybernetic modeling of bacterial cultures at low growth rates: mixed-substrate system Biotechnol Bioeng 32 46–54 Occurrence Handle10.1002/bit.260320108
J Liebig Particlevon (1840) Organic Chemistry, Its Application to Agriculture. Physiology (English translation by L Playfair Taylor) Walton London
HV Westerhoff W Heeswijk Particlevan D Kahn DB Kell (1991) ArticleTitleQuantitative approaches to the analysis of the control, regulation of microbial metabolism Antonie van Leeuwenhoek 60 193–207 Occurrence Handle10.1007/BF00430365 Occurrence Handle1687235
Acknowledgments
Interest in this paper was generated during conversations with Atul Narang when he was part of the Ramkrishna group at Purdue University. The approach and experiments of Tom Egli and his group in Zurich, Switzerland, is important from a quite different point of view and greatly influenced my thinking. My original interest in bacterial growth came from earlier experiments in the laboratories of Jacque Monod and Adam Kepes at the Institut Pasteur, in whose group I worked during a sabbatical 45 years ago on the transport of galactosides into Escherichia coli. Finally, for their contribution to my thinking I should like to acknowledge my associates and graduate students: Nick Peterson, Bob Coffman, Gayle Gross, Tom Norris, David Nickens, Paul Demchick, Suzanne Pinette, Elio Schaechter, David White, and George Hegeman.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix


Rights and permissions
About this article
Cite this article
Koch, A. Bacterial Choices for the Consumption of Multiple Resources for Current and Future Needs. Microb Ecol 49, 183–197 (2005). https://doi.org/10.1007/s00248-003-1053-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00248-003-1053-4