Abstract
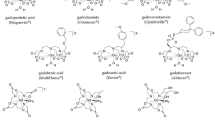
Gadolinium-based contrast agents (GBCAs) have been used to improve image quality of MRI examinations for decades and have an excellent overall safety record. However, there are well-documented risks associated with GBCAs and our understanding and management of these risks continue to evolve. The purpose of this review is to discuss the safety of GBCAs used in MRI in adult and pediatric populations. We focus particular attention on acute adverse reactions, nephrogenic systemic fibrosis and gadolinium deposition. We also discuss the non-GBCA MRI contrast agent ferumoxytol, which is increasing in use and has its own risk profile. Finally, we identify special populations at higher risk of harm from GBCA administration.

Similar content being viewed by others
References
Lohrke J, Frenzel T, Endrikat J et al (2016) 25 years of contrast-enhanced MRI: developments, current challenges and future perspectives. Adv Ther 33:1–28
Runge VM, Stewart RG, Clanton JA et al (1983) Work in progress: potential oral and intravenous paramagnetic NMR contrast agents. Radiology 147:789–791
Mathur M, Jones JR, Weinreb JC (2020) Gadolinium deposition and nephrogenic systemic fibrosis: a radiologist's primer. Radiographics 40:153–162
Balzer T (2017) Presence of gadolinium (Gd) in the brain and body. Presentation to the medical imaging drugs advisory committee. United States Food and Drug Administration, Silver Spring
Bleicher AG, Kanal E (2008) Assessment of adverse reaction rates to a newly approved MRI contrast agent: review of 23,553 administrations of gadobenate dimeglumine. AJR Am J Roentgenol 191:W307–W311
Ramalho J, Semelka RC, Ramalho M et al (2016) Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol 37:1192–1198
Grobner T (2006) Gadolinium — a specific trigger for the development of nephrogenic fibrosing dermopathy and nephrogenic systemic fibrosis? Nephrol Dial Transplant 21:1104–1108
Marckmann P, Skov L, Rossen K et al (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17:2359–2362
Kanda T, Ishii K, Kawaguchi H et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
Errante Y, Cirimele V, Mallio CA et al (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49:685–690
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Holowka S, Shroff M, Chavhan GB (2019) Use and safety of gadolinium based contrast agents in pediatric MR imaging. Indian J Pediatr 86:961–966
Mitsumori LM, Bhargava P, Essig M, Maki JH (2014) Magnetic resonance imaging using gadolinium-based contrast agents. Top Magn Reson Imaging 23:51–69
Kanda T, Oba H, Toyoda K et al (2016) Brain gadolinium deposition after administration of gadolinium-based contrast agents. Jpn J Radiol 34:3–9
Rogosnitzky M, Branch S (2016) Gadolinium-based contrast agent toxicity: a review of known and proposed mechanisms. Biometals 29:365–376
Frenzel T, Lengsfeld P, Schirmer H et al (2008) Stability of gadolinium-based magnetic resonance imaging contrast agents in human serum at 37 degrees C. Investig Radiol 43:817–828
Fraum TJ, Ludwig DR, Bashir MR, Fowler KJ (2017) Gadolinium-based contrast agents: a comprehensive risk assessment. J Magn Reson Imaging 46:338–353
Juluru K, Vogel-Claussen J, Macura KJ et al (2009) MR imaging in patients at risk for develo** nephrogenic systemic fibrosis: protocols, practices, and imaging techniques to maximize patient safety. Radiographics 29:9–22
Murphy A, Morgan M (n.d.) Gadofosveset trisodium. Radiopaedia website. https://radiopaedia.org/articles/gadofosveset-trisodium-1?lang=us. Accessed 1 Sep 2020
Llamas M (2020) Gadolinium. Drugwatch website. https://www.drugwatch.com/gadolinium/. Accessed 1 Sep 2020
Bayer HealthCare (2019) [Letter to customer]. Bayer in radiology website. https://www.radiologysolutions.bayer.com/sites/g/files/kmftyc641/files/MV%20EOS%20Letter%20-%20GPO%20PDF%20R8v1.pdf. Accessed 1 Sep 2020
American College of Radiology (2020) Manual on contrast media, Version 10.3. ACR website. https://www.acr.org/Clinical-Resources/Contrast-Manual. Accessed 16 May 2020
Wang YX, Hussain SM, Krestin GP (2001) Superparamagnetic iron oxide contrast agents: physicochemical characteristics and applications in MR imaging. Eur Radiol 11:2319–2331
Pai AB, Garba AO (2012) Ferumoxytol: a silver lining in the treatment of anemia of chronic kidney disease or another dark cloud? J Blood Med 3:77–85
Li W, Tutton S, Vu AT et al (2005) First-pass contrast-enhanced magnetic resonance angiography in humans using ferumoxytol, a novel ultrasmall superparamagnetic iron oxide (USPIO)-based blood pool agent. J Magn Reson Imaging 21:46–52
Prince MR, Zhang HL, Chabra SG et al (2003) A pilot investigation of new superparamagnetic iron oxide (ferumoxytol) as a contrast agent for cardiovascular MRI. J Xray Sci Technol 11:231–240
Ruangwattanapaisarn N, Hsiao A, Vasanawala SS (2015) Ferumoxytol as an off-label contrast agent in body 3T MR angiography: a pilot study in children. Pediatr Radiol 45:831–839
Luhar A, Khan S, Finn JP et al (2016) Contrast-enhanced magnetic resonance venography in pediatric patients with chronic kidney disease: initial experience with ferumoxytol. Pediatr Radiol 46:1332–1340
Toth GB, Varallyay CG, Horvath A et al (2017) Current and potential imaging applications of ferumoxytol for magnetic resonance imaging. Kidney Int 92:47–66
Hope MD, Hope TA, Zhu C et al (2015) Vascular imaging with ferumoxytol as a contrast agent. AJR Am J Roentgenol 205:W366–W373
Muehe AM, Siedek F, Theruvath AJ et al (2020) Differentiation of benign and malignant lymph nodes in pediatric patients on ferumoxytol-enhanced PET/MRI. Theranostics 10:3612–3621
Storey P, Lim RP, Chandarana H et al (2012) MRI assessment of hepatic iron clearance rates after USPIO administration in healthy adults. Investig Radiol 47:717–724
Dillman JR, Trout AT, Davenport MS (2018) Allergic-like contrast media reaction management in children. Pediatr Radiol 48:1688–1694
Behzadi AH, Zhao Y, Farooq Z, Prince MR (2018) Immediate allergic reactions to gadolinium-based contrast agents: a systematic review and meta-analysis. Radiology 286:731
McDonald JS, Hunt CH, Kolbe AB et al (2019) Acute adverse events following gadolinium-based contrast agent administration: a single-center retrospective study of 281,945 injections. Radiology 292:620–627
Dillman JR, Ellis JH, Cohan RH et al (2007) Frequency and severity of acute allergic-like reactions to gadolinium-containing i.v. contrast media in children and adults. AJR Am J Roentgenol 189:1533–1538
Forbes-Amrhein MM, Dillman JR, Trout AT et al (2018) Frequency and severity of acute allergic-like reactions to intravenously administered gadolinium-based contrast media in children. Investig Radiol 53:313–318
Walker DT, Davenport MS, McGrath TA et al (2020) Breakthrough hypersensitivity reactions to gadolinium-based contrast agents and strategies to decrease subsequent reaction rates: a systematic review and meta-analysis. Radiology 296:312–321
Blumfield E, Moore MM, Drake MK et al (2017) Survey of gadolinium-based contrast agent utilization among the members of the Society for Pediatric Radiology: a quality and safety committee report. Pediatr Radiol 47:665–673
Schiller B, Bhat P, Sharma A (2014) Safety and effectiveness of ferumoxytol in hemodialysis patients at 3 dialysis chains in the United States over a 12-month period. Clin Ther 36:70–83
Varallyay CG, Nesbit E, Horvath A et al (2018) Cerebral blood volume map** with ferumoxytol in dynamic susceptibility contrast perfusion MRI: comparison to standard of care. J Magn Reson Imaging 48:441–448
Vasanawala SS, Nguyen KL, Hope MD et al (2016) Safety and technique of ferumoxytol administration for MRI. Magn Reson Med 75:2107–2111
Muehe AM, Feng D, von Eyben R et al (2016) Safety report of ferumoxytol for magnetic resonance imaging in children and young adults. Investig Radiol 51:221–227
Cowper SE, Bucala R, Leboit PE (2006) Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis — setting the record straight. Semin Arthritis Rheum 35:208–210
Swaminathan S, Horn TD, Pellowski D et al (2007) Nephrogenic systemic fibrosis, gadolinium, and iron mobilization. N Engl J Med 357:720–722
Wagner B, Tan C, Barnes JL et al (2012) Nephrogenic systemic fibrosis: evidence for oxidative stress and bone marrow-derived fibrocytes in skin, liver, and heart lesions using a 5/6 nephrectomy rodent model. Am J Pathol 181:1941–1952
Attari H, Cao Y, Elmholdt TR et al (2019) A systematic review of 639 patients with biopsy-confirmed nephrogenic systemic fibrosis. Radiology 292:376–386
Jimenez SA, Artlett CM, Sandorfi N et al (2004) Dialysis-associated systemic fibrosis (nephrogenic fibrosing dermopathy): study of inflammatory cells and transforming growth factor beta1 expression in affected skin. Arthritis Rheum 50:2660–2666
Daram SR, Cortese CM, Bastani B (2005) Nephrogenic fibrosing dermopathy/nephrogenic systemic fibrosis: report of a new case with literature review. Am J Kidney Dis 46:754–759
Sadowski EA, Bennett LK, Chan MR et al (2007) Nephrogenic systemic fibrosis: risk factors and incidence estimation. Radiology 243:148–157
Wertman R, Altun E, Martin DR et al (2008) Risk of nephrogenic systemic fibrosis: evaluation of gadolinium chelate contrast agents at four American universities. Radiology 248:799–806
Abu-Alfa AK (2011) Nephrogenic systemic fibrosis and gadolinium-based contrast agents. Adv Chronic Kidney Dis 18:188–198
Prince MR, Zhang H, Morris M et al (2008) Incidence of nephrogenic systemic fibrosis at two large medical centers. Radiology 248:807–816
Collidge TA, Thomson PC, Mark PB et al (2007) Gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis: retrospective study of a renal replacement therapy cohort. Radiology 245:168–175
Nardone B, Saddleton E, Laumann AE et al (2014) Pediatric nephrogenic systemic fibrosis is rarely reported: a RADAR report. Pediatr Radiol 44:173–180
Abu Alfa AK (2010) Approach to the use of GBCA in patients with kidney disease. Presented at the fourth annual Symposium on Nephrogenic Systemic Fibrosis and Gadolinium-based Contrast Agents, May 14-15, 2010, New York
Elmholdt TR, Pedersen M, Jorgensen B et al (2011) Nephrogenic systemic fibrosis is found only among gadolinium-exposed patients with renal insufficiency: a case-control study from Denmark. Br J Dermatol 165:828–836
Swaminathan S, Shah SV (2007) New insights into nephrogenic systemic fibrosis. J Am Soc Nephrol 18:2636–2643
United States Food and Drug Administration (2011) Gadolinium-based contrast agents (GBCAs) and the NSF risk: regulatory update. Online report. https://wayback.archive-it.org/7993/20170405225258/https://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PeripheralandCentralNervousSystemDrugsAdvisoryCommittee/UCM241072.pdf. Accessed 14 April 2020
European Medicines Agency (2010) Assessment report for gadolinium-containing contrast agents. https://www.ema.europa.eu/docs/en_GB/document_library/Referrals_document/gadolinium_31/WC500099538.pdf. Online report. Accessed 14 April 2020
Schieda N, van der Pol CB, Walker D et al (2020) Adverse events to the gadolinium-based contrast agent gadoxetic acid: systematic review and meta-analysis. Radiology. https://doi.org/10.1148/radiol.2020200073
Kirchin MA, Lorusso V, Pirovano G (2015) Compensatory biliary and urinary excretion of gadobenate ion after administration of gadobenate dimeglumine (MultiHance) in cases of impaired hepatic or renal function: a mechanism that may aid in the prevention of nephrogenic systemic fibrosis? Br J Radiol 88:20140526
Prince MR, Zhang HL, Roditi GH et al (2009) Risk factors for NSF: a literature review. J Magn Reson Imaging 30:1298–1308
Othersen JB, Maize JC, Woolson RF, Budisavljevic MN (2007) Nephrogenic systemic fibrosis after exposure to gadolinium in patients with renal failure. Nephrol Dial Transplant 22:3179–3185
McWilliams RG, Frabizzio JV, De Backer AI et al (2020) Observational study on the incidence of nephrogenic systemic fibrosis in patients with renal impairment following gadoterate meglumine administration: the NSsaFe study. J Magn Reson Imaging 51:607–614
Deray G, Rouviere O, Bacigalupo L et al (2013) Safety of meglumine gadoterate (Gd-DOTA)-enhanced MRI compared to unenhanced MRI in patients with chronic kidney disease (RESCUE study). Eur Radiol 23:1250–1259
Janus N, Launay-Vacher V, Karie S et al (2010) Prevalence of nephrogenic systemic fibrosis in renal insufficiency patients: results of the FINEST study. Eur J Radiol 73:357–359
Amet S, Launay-Vacher V, Clement O et al (2014) Incidence of nephrogenic systemic fibrosis in patients undergoing dialysis after contrast-enhanced magnetic resonance imaging with gadolinium-based contrast agents: the prospective Fibrose Nephrogenique Systemique study. Investig Radiol 49:109–115
Franano FN, Edwards WB, Welch MJ et al (1995) Biodistribution and metabolism of targeted and nontargeted protein-chelate-gadolinium complexes: evidence for gadolinium dissociation in vitro and in vivo. Magn Reson Imaging 13:201–214
Gibby WA, Gibby KA, Gibby WA (2004) Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Investig Radiol 39:138–142
Sieber MA, Lengsfeld P, Frenzel T et al (2008) Preclinical investigation to compare different gadolinium-based contrast agents regarding their propensity to release gadolinium in vivo and to trigger nephrogenic systemic fibrosis-like lesions. Eur Radiol 18:2164–2173
Maximova N, Gregori M, Zennaro F et al (2016) Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology 281:418–426
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd(DTPA-BMA) (Omniscan) versus Gd(HP-DO3A) (ProHance) relative to gadolinium retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig Radiol 41:272–278
**a D, Davis RL, Crawford JA, Abraham JL (2010) Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive X-ray spectroscopy. Acta Radiol 51:1126–1136
McDonald RJ, McDonald JS, Kallmes DF et al (2017) Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology 285:546–554
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
McDonald RJ, Levine D, Weinreb J et al (2018) Gadolinium retention: a research roadmap from the 2018 NIH/ACR/RSNA workshop on gadolinium chelates. Radiology 289:517–534
McDonald JS, McDonald RJ, Jentoft ME et al (2017) Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: a case-control study. JAMA Pediatr 171:705–707
Stanescu AL, Shaw DW, Murata N et al (2020) Brain tissue gadolinium retention in pediatric patients after contrast-enhanced magnetic resonance exams: pathological confirmation. Pediatr Radiol 50:388–396
Zhang Y, Cao Y, Shih GL et al (2017) Extent of signal hyperintensity on unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology 282:516–525
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Radbruch A, Haase R, Kieslich PJ et al (2017) No signal intensity increase in the dentate nucleus on unenhanced T1-weighted MR images after more than 20 serial injections of macrocyclic gadolinium-based contrast agents. Radiology 282:699–707
Lee JY, Park JE, Kim HS et al (2017) Up to 52 administrations of macrocyclic ionic MR contrast agent are not associated with intracranial gadolinium deposition: multifactorial analysis in 385 patients. PLoS One 12:e0183916
Tibussek D, Rademacher C, Caspers J et al (2017) Gadolinium brain deposition after macrocyclic gadolinium administration: a pediatric case-control study. Radiology 285:223–230
Ryu YJ, Choi YH, Cheon JE et al (2018) Pediatric brain: gadolinium deposition in dentate nucleus and globus pallidus on unenhanced T1-weighted images is dependent on the type of contrast agent. Investig Radiol 53:246–255
Renz DM, Kumpel S, Bottcher J et al (2018) Comparison of unenhanced T1-weighted signal intensities within the dentate nucleus and the globus pallidus after serial applications of gadopentetate dimeglumine versus gadobutrol in a pediatric population. Investig Radiol 53:119–127
Murata N, Gonzalez-Cuyar LF, Murata K et al (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Investig Radiol 51:447–453
Robert P, Lehericy S, Grand S et al (2015) T1-weighted hypersignal in the deep cerebellar nuclei after repeated administrations of gadolinium-based contrast agents in healthy rats: difference between linear and macrocyclic agents. Investig Radiol 50:473–480
Nunn AD, Wedeking P, Marinelli E et al (1996) Toxicity of gadolinium chelates in rodents. Acad Radiol 3:S333–S335
Pietsch H, Raschke M, Ellinger-Ziegelbauer H et al (2011) The role of residual gadolinium in the induction of nephrogenic systemic fibrosis-like skin lesions in rats. Investig Radiol 46:48–56
Ray DE, Holton JL, Nolan CC et al (1998) Neurotoxic potential of gadodiamide after injection into the lateral cerebral ventricle of rats. AJNR Am J Neuroradiol 19:1455–1462
Roman-Goldstein SM, Barnett PA, McCormick CI et al (1991) Effects of gadopentetate dimeglumine administration after osmotic blood-brain barrier disruption: toxicity and MR imaging findings. AJNR Am J Neuroradiol 12:885–890
Burke LM, Ramalho M, AlObaidy M et al (2016) Self-reported gadolinium toxicity: a survey of patients with chronic symptoms. Magn Reson Imaging 34:1078–1080
Gulani V, Calamante F, Shellock FG et al (2017) Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 16:564–570
European Medicines Agency (2017) EMA’s final opinion confirms restrictions on use of linear gadolinium agents in body scans. Online report. https://www.ema.europa.eu/en/documents/referral/gadolinium-article-31-referral-emas-final-opinion-confirms-restrictions-use-linear-gadolinium-agents_en.pdf. Accessed 24 May 2020
United States Food and Drug Administration (2017) FDA drug safety communication: FDA identifies no harmful effects to date with brain retention of gadolinium-based contrast agents for MRIs; review to continue. Online report. https://www.fda.gov/downloads/Drugs/DrugSafety/UCM559654.pdf. Accessed 24 May 2020
United States Food and Drug Administration (2018) FDA drug safety communication: FDA warns that gadolinium-based contrast agents (GBCAs) are retained in the body; requires new class warnings. Online report. https://www.fda.gov/media/109825/download. Accessed 24 May 2020
Tirada N, Dreizin D, Khati NJ et al (2015) Imaging pregnant and lactating patients. Radiographics 35:1751–1765
Salomon LJ, Siauve N, Balvay D et al (2005) Placental perfusion MR imaging with contrast agents in a mouse model. Radiology 235:73–80
Palacios Jaraquemada JM, Bruno C (2000) Gadolinium-enhanced MR imaging in the differential diagnosis of placenta accreta and placenta percreta. Radiology 216:610–611
Novak Z, Thurmond AS, Ross PL et al (1993) Gadolinium-DTPA transplacental transfer and distribution in fetal tissue in rabbits. Investig Radiol 28:828–830
Puac P, Rodriguez A, Vallejo C et al (2017) Safety of contrast material use during pregnancy and lactation. Magn Reson Imaging Clin N Am 25:787–797
Webb JA, Thomsen HS, Morcos SK, Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (2005) The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol 15:1234–1240
Tremblay E, Therasse E, Thomassin-Naggara I, Trop I (2012) Quality initiatives: guidelines for use of medical imaging during pregnancy and lactation. Radiographics 32:897–911
Ray JG, Vermeulen MJ, Bharatha A et al (2016) Association between MRI exposure during pregnancy and fetal and childhood outcomes. JAMA 316:952–961
Mathur S, Pillenahalli Maheshwarappa R, Fouladirad S et al (2020) Emergency imaging in pregnancy and lactation. Can Assoc Radiol J 71:396–402
Hotham N, Hotham E (2015) Drugs in breastfeeding. Aust Prescr 38:156–159
Sulemanji M, Vakili K (2013) Neonatal renal physiology. Semin Pediatr Surg 22:195–198
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
Dr. Chan has received a research grant and honorarium from Jazz Pharmaceuticals.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ponrartana, S., Moore, M.M., Chan, S.S. et al. Safety issues related to intravenous contrast agent use in magnetic resonance imaging. Pediatr Radiol 51, 736–747 (2021). https://doi.org/10.1007/s00247-020-04896-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-020-04896-7