Abstract
Gadolinium has been used as a base for contrast agents in MRI for the last three decades. Numerous studies over the last 4 years have reported increased signal intensity in deep brain nuclei in non-contrast MRI images following gadolinium-based contrast agent (GBCA) administration. Pathology studies performed on adults and children, and rodent necropsy studies have also shown gadolinium deposition in brain and other tissues after GBCA administration. The purpose of this review was to summarize and discuss the knowledge gained from these reports and the relevance for imaging pediatric patients.
Similar content being viewed by others
References
Idée JM, Port M, Medina C et al (2008) Possible involvement of gadolinium chelates in the pathophysiology of nephrogenic systemic fibrosis: a critical review. Toxicology 248:77–88
Huckle JE, Altun E, Jay M, Semelka RC (2016) Gadolinium deposition in humans: when did we learn that gadolinium was deposited in vivo? Investig Radiol 51:236–240
Blumfield E, Moore MM, Drake MK et al (2017) Survey of gadolinium-based contrast agent utilization among the members of the Society for Pediatric Radiology: a quality and safety committee report. Pediatr Radiol 47:665–673
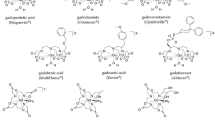
Sherry AD, Caravan P, Lenkinski RE (2009) Primer on gadolinium chemistry. J Magn Reson Imaging 30:1240–1248
Idée JM, Port M, Robic C et al (2009) Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J Magn Reson Imaging 30:1249–1258
Ramalho J, Semelka RC, Ramalho M et al (2016) Gadolinium-based contrast agent accumulation and toxicity: an update. AJNR Am J Neuroradiol 37:1192–1198
Grobner T, Prischl FC (2008) Patient characteristics and risk factors for nephrogenic systemic fibrosis following gadolinium exposure. Semin Dial 21:135–139
Marckmann P (2006) Nephrogenic systemic fibrosis: suspected causative role of gadodiamide used for contrast-enhanced magnetic resonance imaging. J Am Soc Nephrol 17:2359–2362
Davenport MS, Asch D, Cavallo J et al (2017) ACR manual on contrast media version 10.3. American College of Radiology Committee on Drugs and Contrast Media. https://www.acr.org/-/media/ACR/Files/Clinical-Resources/Contrast_Media.pdf. Accessed 04 Oct 2018
Kanda T, Ishii K, Kawaguchi H et al (2014) High signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images: relationship with increasing cumulative dose of a gadolinium-based contrast material. Radiology 270:834–841
White GW, Gibby WA, Tweedle MF (2006) Comparison of Gd (DTPA-BMA) (Omniscan) versus retention in human bone tissue by inductively coupled plasma mass spectroscopy. Investig Radiol 41:272–278
Tweedle MF (1992) Physicochemical properties of gadoteridol and other magnetic resonance contrast agents. Investig Radiol 27:S2–S6
Errante Y, Cirimele V, Mallio CA et al (2014) Progressive increase of T1 signal intensity of the dentate nucleus on unenhanced magnetic resonance images is associated with cumulative doses of intravenously administered gadodiamide in patients with normal renal function, suggesting dechelation. Investig Radiol 49:685–690
Zhang Y, Cao Y, Shih GL et al (2017) Extent of signal hyperintensity on unenhanced T1-weighted brain MR images after more than 35 administrations of linear gadolinium-based contrast agents. Radiology 282:516–525
Weberling LD, Kieslich PJ, Kickingereder P et al (2015) Increased signal intensity in the dentate nucleus on unenhanced T1-weighted images after gadobenate dimeglumine administration. Investig Radiol 50:743–748
Ramalho J, Castillo M, AlObaidy M et al (2015) High signal intensity in globus pallidus and dentate nucleus on unenhanced T1-weighted MR images: evaluation of two linear gadolinium-based contrast agents. Radiology 276:836–844
Conte G, Preda L, Cocorocchio E et al (2017) Signal intensity change on unenhanced T1-weighted images in dentate nucleus and globus pallidus after multiple administrations of gadoxetate disodium: an intraindividual comparative study. Eur Radiol 27:4372–4378
Kanda T, Osawa M, Oba H et al (2015) High signal intensity in dentate nucleus on unenhanced T1-weighted MR images: association with linear versus macrocyclic gadolinium chelate administration. Radiology 275:803–809
Radbruch A, Weberling LD, Kieslich PJ et al (2015) Gadolinium retention in the dentate nucleus and globus pallidus is dependent on the class of contrast agent. Radiology 275:783–791
Cao Y, Huang DQ, Shih G, Prince MR (2016) Signal change in the dentate nucleus on T1-weighted MR images after multiple administrations of gadopentetate dimeglumine versus gadobutrol. AJR Am J Roentgenol 206:414–419
Bae S, Lee HJ, Han K et al (2017) Gadolinium deposition in the brain: association with various GBCAs using a generalized additive model. Eur Radiol 27:3353–3361
Radbruch A, Weberling LD, Kieslich PJ et al (2015) High-signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evaluation of the macrocyclic gadolinium-based contrast agent gadobutrol. Investig Radiol 50:805–810
Langner S, Kromrey ML, Kuehn JP et al (2017) Repeated intravenous administration of gadobutrol does not lead to increased signal intensity on unenhanced T1-weighted images — a voxel-based whole brain analysis. Eur Radiol 27:3687–3693
Kromrey ML, Liedtke KR, Ittermann T et al (2017) Intravenous injection of gadobutrol in an epidemiological study group did not lead to a difference in relative signal intensities of certain brain structures after 5 years. Eur Radiol 27:772–778
Lee JY, Park JE, Kim HS et al (2017) Up to 52 administrations of macrocyclic ionic MR contrast agent are not associated with intracranial gadolinium deposition: multifactorial analysis in 385 patients. PLoS One 12:1–15
Bjørnerud A, Vatnehol SAS, Larsson C et al (2017) Signal enhancement of the dentate nucleus at unenhanced MR imaging after very high cumulative doses of the macrocyclic gadolinium-based contrast agent gadobutrol: an observational study. Radiology 285:170391
Forslin Y, Shams S, Hashim F et al (2017) Retention of gadolinium-based contrast agents in multiple sclerosis: retrospective analysis of an 18-year longitudinal study. AJNR Am J Neuroradiol 38:1311–1316
Stojanov DA, Aracki-Trenkic A, Vo**ovic S et al (2016) Increasing signal intensity within the dentate nucleus and globus pallidus on unenhanced T1W magnetic resonance images in patients with relapsing-remitting multiple sclerosis: correlation with cumulative dose of a macrocyclic gadolinium-based contrast agent, gadobutrol. Eur Radiol 26:807–815
Splendiani A, Perri M, Marsecano C et al (2018) Effects of serial macrocyclic-based contrast materials gadoterate meglumine and gadobutrol administrations on gadolinium-related dentate nuclei signal increases in unenhanced T1-weighted brain: a retrospective study in 158 multiple sclerosis (MS) patients. Radiol Med 123:125–134
Gibby WA, Gibby KA, Gibby WA (2004) Comparison of Gd DTPA-BMA (Omniscan) versus Gd HP-DO3A (ProHance) retention in human bone tissue by inductively coupled plasma atomic emission spectroscopy. Investig Radiol 39:138–142
Darrah TH, Prutsman-Pfeiffer JJ, Poreda RJ et al (2009) Incorporation of excess gadolinium into human bone from medical contrast agents. Metallomics 1:479–488
**a D, Davis RL, Crawford JA, Abraham JL (2010) Gadolinium released from MR contrast agents is deposited in brain tumors: in situ demonstration using scanning electron microscopy with energy dispersive X-ray spectroscopy. Acta Radiol 51:1126–1136
Christensen KN, Lee CU, Hanley MM et al (2011) Quantification of gadolinium in fresh skin and serum samples from patients with nephrogenic systemic fibrosis. J Am Acad Dermatol 64:91–96
McDonald RJ, McDonald JS, Kallmes DF et al (2015) Intracranial gadolinium deposition after contrast-enhanced MR imaging. Radiology 275:772–782
Kanda T, Fukusato T, Matsuda M et al (2015) Gadolinium-based contrast agent accumulates in the brain even in subjects without severe renal dysfunction: evaluation of autopsy brain specimens with inductively coupled plasma mass spectroscopy. Radiology 276:228–232
McDonald RJ, McDonald JS, Kallmes DF et al (2017) Gadolinium deposition in human brain tissues after contrast-enhanced MR imaging in adult patients without intracranial abnormalities. Radiology 285:161595
Murata N, Gonzalez-Cuyar LF, Murata K et al (2016) Macrocyclic and other non-group 1 gadolinium contrast agents deposit low levels of gadolinium in brain and bone tissue: preliminary results from 9 patients with normal renal function. Investig Radiol 51:447–453
McDonald JS, McDonald RJ, Jentoft ME et al (2017) Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: a case-control study. JAMA Pediatr 171:705–707
Balassy C, Roberts D, Miller SF (2015) Safety and efficacy of gadoteric acid in pediatric magnetic resonance imaging: overview of clinical trials and post-marketing studies. Pediatr Radiol 45:1831–1841
Ball WS, Nadel SN, Zimmerman RA et al (1993) Phase III multicenter clinical investigation to determine the safety and efficacy of godoteridol in children suspected of having neurologic disease. Radiology 186:769–774
Elster AD (1990) Cranial MR imaging with Gd-DTPA in neonates and young infants: preliminary experience. Radiology 176:225–230
Hahn G, Sorge I, Hirsch W et al (2009) Pharmacokinetics and safety of gadobutrol-enhanced magnetic resonance imaging in pediatric patients. Investig Radiol 44:776–783
Lundby B, Gordon P, Hugo F (1996) MRI in children given gadodiamide injection: safety and efficacy in CNS and body indications. Eur J Radiol 23:190–196
Schneider G, Schürholz H (2013) Safety and adverse effects during 24 hours after contrast-enhanced MRI with gadobenate dimeglumine (MultiHance) in children. Pediatri Radiol 43:202–211
Gale EM, Caravan P, Rao AG et al (2017) Gadolinium-based contrast agents in pediatric magnetic resonance imaging. Pediatr Radiol 47:507–521
Roberts DR, Chatterjee AR, Yazdani M et al (2016) Pediatric patients demonstrate progressive T1-weighted hyperintensity in the dentate nucleus following multiple doses of gadolinium-based contrast agent. AJNR Am J Neuroradiol 37:2340–2347
Mendichovszky IA, Marks SD, Simcock CM, Olsen ØE (2008) Gadolinium and nephrogenic systemic fibrosis: time to tighten practice. Pediatr Radiol 38:489–496
Miller JH, Hu HH, Pokorney A et al (2015) MRI brain signal intensity changes of a child during the course of 35 gadolinium contrast examinations. Pediatrics 136:e1637–e1640
Roberts DR, Holden KR (2016) Progressive increase of T1 signal intensity in the dentate nucleus and globus pallidus on unenhanced T1-weighted MR images in the pediatric brain exposed to multiple doses of gadolinium contrast. Brain Dev 38:331–336
Hu HH, Pokorney A, Towbin RB, Miller JH (2016) Increased signal intensities in the dentate nucleus and globus pallidus on unenhanced T1-weighted images: evidence in children undergoing multiple gadolinium MRI exams. Pediatr Radiol 46:1590–1598
Kasper E, Schemuth HP, Horry S, Kinner S (2018) Changes in signal intensity in the dentate nucleus at unenhanced T1-weighted magnetic resonance imaging depending on class of previously used gadolinium-based contrast agent. Pediatr Radiol 48:686–693
Flood TF, Stence NV, Maloney JA, Mirsky DM (2017) Pediatric brain: repeated exposure to linear gadolinium-based contrast material is associated with increased signal intensity at unenhanced T1-weighted MR imaging. Radiology 282:222–228
Schneider GK, Stroeder J, Roditi G et al (2017) T1 signal measurements in pediatric brain: findings after multiple exposures to gadobenate dimeglumine for imaging of nonneurologic disease. AJNR Am J Neuroradiol 38:1799–1806
Radbruch A, Haase R, Kickingereder P et al (2017) Pediatric brain: no increased signal intensity in the dentate nucleus on unenhanced T1-weighted MR images after consecutive exposure to a macrocyclic gadolinium-based contrast agent. Radiology 283:828–836
Tibussek D, Rademacher C, Caspers J et al (2017) Gadolinium brain deposition after macrocyclic gadolinium administration: a pediatric case-control study. Radiology 285:223–230
Rossi Espagnet MC, Bernardi B, Pasquini L et al (2017) Signal intensity at unenhanced T1-weighted magnetic resonance in the globus pallidus and dentate nucleus after serial administrations of a macrocyclic gadolinium-based contrast agent in children. Pediatr Radiol 47:1345–1352
Radbruch A, Quattrocchi CC (2017) Interpreting signal-intensity ratios without visible T1 hyperintensities in clinical gadolinium retention studies. Pediatr Radiol 47:1688–1689
Rossi-Espagnet MC, Tomà P, Napolitano A (2017) Reply to Radbruch et al.: ‘interpreting signal-intensity ratios without visible T1 hyperintensities in clinical gadolinium retention studies.’ Pediatr Radiol 47:1690–1691
Tamrazi B, Nguyen B, C-SJ L et al (2017) Changes in signal intensity of the dentate nucleus and globus pallidus in pediatric patients: impact of brain irradiation and presence of primary brain tumors independent of linear gadolinium-based contrast agent administration. Radiology 287:171850
Mithal LB, Patel PS, Mithal D et al (2017) Use of gadolinium-based magnetic resonance imaging contrast agents and awareness of brain gadolinium deposition among pediatric providers in North America. Pediatr Radiol 47:657–664
Maximova N, Gregori M, Zennaro F et al (2016) Hepatic gadolinium deposition and reversibility after contrast agent-enhanced MR imaging of pediatric hematopoietic stem cell transplant recipients. Radiology 281:418–426
Roberts DR, Welsh CA, LeBel II DP, Davis WC (2017) Distribution map of gadolinium deposition within the cerebellum following GBCA administration. Neurology 88:1206–1208
McDonald RJ, McDonald JS, Dai D et al (2017) Comparison of gadolinium concentrations within multiple rat organs after intravenous administration of linear versus macrocyclic gadolinium chelates. Radiology 285:161594
Gianolio E, Bardini P, Arena F et al (2017) Gadolinium retention in the rat brain: assessment of the amounts of insoluble gadolinium-containing species and intact gadolinium complexes after repeated administration of gadolinium-based contrast agents. Radiology 285:839–849
Frenzel T, Apte C, Jost G et al (2017) Quantification and assessment of the chemical form of residual gadolinium in the brain after repeated administration of gadolinium-based contrast agents: comparative study in rats. Investig Radiol 52:396–404
Kartamihardja AAP, Nakajima T, Kameo S et al (2016) Distribution and clearance of retained gadolinium in the brain: differences between linear and macrocyclic gadolinium based contrast agents in a mouse model. Br J Radiol 89:20160509
Wedeking P, Kumar K, Tweedle MF (1992) Dissociation of gadolinium chelates in mice: relationship to chemical characteristics. Magn Reson Imaging 10:641–648
Bussi S, Coppo A, Botteron C et al (2018) Differences in gadolinium retention after repeated injections of macrocyclic MR contrast agents to rats. J Magn Reson Imaging 47:746–752
Ramalho M, Ramalho J, Burke LM, Semelka RC (2017) Gadolinium retention and toxicity — an update. Adv Chronic Kidney Dis 24:138–146
Murata N, Murata K, Gonzalez-Cuyar LF, Maravilla KR (2016) Gadolinium tissue deposition in brain and bone. Magn Reson Imaging 34:1359–1365
Ramalho J, Ramalho M (2017) Gadolinium deposition and chronic toxicity. Magn Reson Imaging Clin N Am 25:765–778
Semelka RC, Ramalho M, AlObaidy M, Ramalho J (2016) Gadolinium in humans: a family of disorders. AJR Am J Roentgenol 207:229–233
RSNA Daily Bulletin (2017) No evidence gadolinium causes neurologic harm. Radiological Society of North America. https://rsna2017.rsna.org/dailybulletin/index.cfm?pg=17fri10. Accessed 04 Oct 2018
Goske MJ, Applegate KE, Boylan J et al (2008) The image gently campaign: working together to change practice. AJR Am J Roentgenol 190:273–274
Scheinfeld MH, Moon JY, Fagan MJ et al (2017) MRI usage in a pediatric emergency department: an analysis of usage and usage trends over 5 years. Pediatr Radiol 47:327–332
Maloney E, Stanescu AL, Perez FA et al (2018) Surveillance magnetic resonance imaging for isolated optic pathway gliomas: is gadolinium necessary? Pediatr Radiol 48:1472–1484
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Blumfield, E., Swenson, D.W., Iyer, R.S. et al. Gadolinium-based contrast agents — review of recent literature on magnetic resonance imaging signal intensity changes and tissue deposits, with emphasis on pediatric patients. Pediatr Radiol 49, 448–457 (2019). https://doi.org/10.1007/s00247-018-4304-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-018-4304-8