Abstract
Purpose
Gamma-hydroxybutyrate (GHB) withdrawal is a life-threatening condition that does not always respond to standard treatment with benzodiazepines. Baclofen has potential utility as a pharmacological adjunct and anecdotal reports suggest that it is being used by drug users to self-manage GHB withdrawal symptoms. Here, we investigate current patterns of use and the online availably of baclofen.
Methods
Data triangulation techniques were applied to published scientific literature and publicly accessible Internet resources (grey literature) to assess the use of baclofen in GHB withdrawal. An Internet snapshot survey was performed to identify the availability of baclofen for online purchase and the compliance of retailers with the UK regulations. Data were collected according to pre-defined criteria.
Results
A total of 37 cases of baclofen use in GHB withdrawal were identified in the scientific literature, as well as 51 relevant discussion threads across eight Internet forums in the grey literature. Baclofen was available to purchase from 38 online pharmacies, of which only one conformed to the UK regulations.
Conclusions
There is limited published evidence on the use of baclofen in GHB withdrawal, but both scientific and grey literature suggests clinical utility. Online pharmacies are readily offering prescription-only-medication without prescription and due to inadequate regulation, pose a danger to the public.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gamma-hydroxybutyrate (GHB) is a naturally occurring metabolite of the neurotransmitter gamma-aminobutyric acid (GABA) and was first synthesised as an anaesthetic agent in 1964 [1]. A high incidence of adverse effects during early clinical trials restricted its use in anaesthesia, but at lower doses, it has found some clinically utility and is licenced for treating sleep disorders (particularly narcolepsy with cataplexy) and alcohol dependence [2]. In addition, it has been used illicitly in both bodybuilding and as a recreational drug [3, 4].
Use of GHB and its precursors (gamma-butryolactone (GBL) and 1,4-butanediol (1,4-BD)) have a dose-dependent effect on glutamate and dopamine release. At low dose, stimulation of GHB receptors increases dopamine release resulting in stimulant-like effects, whilst at higher doses, binding to GABA-B receptors results in hypnotic effects and eventually coma [5]. There is no specific antidote for GHB and so acute toxicity is managed by supportive care which, in the case of significant consumption, may include endotracheal intubation for airway protection [6].
The first reports of physical dependence and consequent withdrawal syndrome were the result of both supervised clinical and unsupervised recreational use [7,8,9]. Dependence results from modulation of baseline neuronal excitability in a mechanism that is analogous to that seen with alcohol and benzodiazepines dependence [10]. A terminal half-life of approximately 40 min means that those with dependency need to dose GHB/GBL every few hours [11], and interruption to this schedule results in a withdrawal syndrome typified by sympathetic over activity, florid delirium and profound neuropsychiatric features [6, 12]. Standard treatment involves high dose benzodiazepines (often up to 200 mg/day diazepam equivalent) with the possible addition of a range of other drugs including antipyschotics, barbiturates and anticonvulsants [13]. Benzodiazepines however are positive allosteric modulators of GABA-A receptors rather than GABA-B where GHB acts, and so despite their action to maintain GABAergic stimulus and prevent harmful neuronal excitability, they may not be the ideal pharmacotherapy [14]. It has therefore been suggested that the use of specific GABA-B agonists such as baclofen may provide clinical benefit in the management of withdrawal beyond that offered by benzodiazepines [6].
Baclofen is a direct agonist at GABA-B receptors [15] and has been widely used for the control of spasticity since the 1960s, where it acts to inhibit spinal afferent pathways and reduce motor neurone activity [16]. Animal work in both small mammals and primates has demonstrated that chronic GHB administration induces cross tolerance to baclofen [17, 18], and one case report described the use of baclofen as rescue therapy when increasing benzodiazepine dose failed to control GHB withdrawal [19].
Anecdotal reports from inpatients treated for GHB/GBL dependence and withdrawal describe dependent users purchasing baclofen from the Internet to self-medicate. The primary aim of this work was to understand and catalogue the use of baclofen in the management of withdrawal from GHB and its precursors. Triangulation of data from published scientific literature and publicly accessible Internet resources has enabled us to address the following objectives: (i) review scientific literature for reports of baclofen use by healthcare professionals, (ii) review Internet forums for reports of baclofen use by the public and (iii) assess the availability of baclofen to purchase online.
Methods
Scientific literature
MEDLINE/PubMed was searched up until the 7th of February 2017 for all articles containing the keyword ‘baclofen’. The same keyword was used to search TOXLINE and EMBASE (up until the 12th of October 2017) and the abstracts of the European Association of Poisons Centres and Clinical Toxicologists (EAPCCT) and North American Congress of Clinical Toxicology (NACCT) congresses (from 2002 to 2016). All potentially eligible manuscripts were reviewed in full to identify those that investigated the use of baclofen in the treatment of GHB/GBL withdrawal. A secondary search was performed on the references of all eligible manuscripts to identify further relevant publications.
Grey literature
Publicly accessible Internet resources were searched in March 2017 for descriptions of baclofen being used to manage GHB/GBL withdrawal. The search term ‘baclofen’ was entered into the Internet search engine google.co.uk with the search terms ‘GHB’, ‘GBL’ and ‘withdrawal’ added separately and in combination. Search results for each combination of search terms were reviewed sequentially until 20 successive irrelevant or duplicate websites were visited. The search terms were then entered into individual discussion forums and blogs identified through the search, as well as searching relevant forums and blogs which had been identified through previous work [20]. Reports of baclofen use to manage GHB/GBL withdrawal were analysed manually and data relating to dosing regimen and adjunct medication were recorded.
Internet snapshot survey
An Internet snapshot survey for the availability of baclofen was undertaken on the 7th of February 2017 using methodology developed by the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) [21]. The term ‘buy baclofen’ was entered into the Internet search engine google.co.uk and approximately 603,000 results were returned. The first 100 search results were reviewed in full followed by sampling to exhaustion until 20 successive websites were defined as being irrelevant, unrelated or a duplication of sites already visited. No baclofen was purchased during this study, but the ability to proceed with an online purchase up to the point of entering financial and/or contact details was accepted as a successful transaction.
Data from each relevant website were recorded according to pre-specified criteria that covered both the pharmaceutical offering and details regarding the online pharmacy. Primary data recorded included (i) form(s) of baclofen available to purchase, (ii) cost, (iii) any requirement for a prescription, (iv) geographical location of the seller and (v) evidence of compliance with United Kingdom (UK) and European Union (EU) registration.
The cost of baclofen was recorded in British Pound Sterling (£) and where alternative currencies were displayed, the European Commission’s online conversion tool was used [22]. Geographical location was recorded according to the first-rank criterion of the provision of a bricks-and-mortar address; subsequently, any statement on the website indicating locations (such as reported adherence to particular laws and regulations) and finally, brand identity. Brand identity was defined as feature of a website that suggested geographical location but can be obtained without a physical presence (i.e. domain name, domain suffix, brand name or telephone number). To further attempt to identify location, a WHOIS query (a tool to find the name and location of the registered domain holder) was performed on each domain [23]. All UK-based online pharmacies are required to be registered with the Medicine and Healthcare products Regulatory Agency (MHRA), and listed on their ‘online medicines seller registry’ [24]. Similarly, all EU-based online pharmacies must be registered with their respective body and display the ‘EU common logo’ on their website (Fig. 1) [25]. The availability of an integrated language translation service, provision of information about baclofen, presence of consumer reviews for baclofen and option to buy in multiple currencies were also recorded.
Ethical considerations
All information recorded from the Internet was obtained from ‘purely public sites’ without the requirement for secure or member access [26]. No identifiable information was recorded when analysing data from the grey literature search. This research was approved by the King’s College London Research Ethics Committee (reference RESCMR-16/17-2097).
Results
Published scientific literature
The literature search identified 4776 unique results and these are summarised in Supplementary Fig. 1. Five relevant publications were identified, consisting of which three were case reports and two clinical study protocols [19, 27,28,29,30]. Two further case reports were identified from abstracts of the NACCT [31, 32], as well as two case series from the Internet search [33, 34], and one case series from the secondary search of references from eligible manuscripts [35].
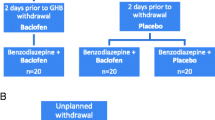
The identified cases (summarised in Table 1) discussed both the use of baclofen as an adjunct for the management of acute GHB withdrawal (n = 37) and the use of baclofen as maintenance therapy to prevent relapse (n = 12). The dose of oral baclofen used to manage withdrawal was 15–60 mg/24 h and in all cases, baclofen was combined with oral or intravenous benzodiazepines. In five cases, baclofen was commenced only when the existing pharmacological approach was felt to be unsuccessful, and the addition of baclofen was reported to be associated with a clinically significant improvement in all of the cases. Clinical outcomes of treatment were not universally reported, and other than one instance of deliberate overdose [36], there were no adverse events attributed to baclofen.
There are no published clinical trials into the efficacy of baclofen in the management and/or maintenance of GHB withdrawal, although our search strategy did identify two study protocols that are yet to report [29, 30].
Grey literature (publicly accessible Internet resources)
Forums
The use of baclofen in GHB withdrawal was identified in threads across eight publicly accessible Internet forums [37,38,39,40,41,42,43,44]. A secondary search within these forums was possible without user registration in six instances, resulting in a total of 51 relevant threads in which the word ‘baclofen’ was mentioned in 138 separate posts. Seven of the forums provided timestamps on posts and the earliest identifiable mention of baclofen use in GHB withdrawal was 2008 [45].
Baclofen dose was mentioned in 16 threads, of which the majority consisted of users posting about their own withdrawal experiences rather than making recommendations for others to follow. The median 24 h baclofen dose was 105 mg (IQR 60–200 mg, range 25–4000 mg). The need to taper medication (and/or GHB/GBL) as part of the withdrawal process was a common theme, but only five provided any form of protocol suggesting how baclofen could be weaned [46,47,48,49,50]. Two of these protocols were the same, contained references to published scientific literature and were credited to NHS Lothian, although the origin of the protocols could not be confirmed [49, 50].
A total of 24 different drugs or drug classes were referred to as adjuncts to, or substitutions for, baclofen in GHB withdrawal. Benzodiazepines were the most mentioned drug class in 32 (62.7%) threads; eight different benzodiazepines were identified including diazepam (16 threads) and chlordiazepoxide (four threads). The next most popular drugs all acted to up-regulate GABA activity: pregabalin (26 threads), phenibut (21 threads) and gabapentin (seven threads). A further 12 drugs were mentioned on a single occasion and these included beta-blockers, antipsychotics and antihistamines. No threads were found to be providing advice on how to obtain medication for GHB withdrawal.
Other relevant websites
In addition to forums, the grey literature search identified a number of websites relevant to the use of baclofen in GHB withdrawal. Two clinical protocols for managing GHB/GBL withdrawal were found, one linking to content from ‘The Maudsley Prescribing Guidelines in Psychiatry’ and the other, a document that appeared to originate from another NHS institution, although this could not be verified [51, 52]. Furthermore, four services were identified that offer assistance with GHB/GBL detoxification, of which one was NHS funded and three located in the USA [53,54,55,56].
Baclofen snapshot survey
The availability of baclofen
Forty-one unique websites selling baclofen online were identified from a total of 195 search results.47 search results involved the addition of a path (e.g. /buy-baclofen.html) to an unrelated web domain so as to facilitate redirection of the Internet user to an online pharmacy. Of these redirections, a single website was the destination in 26 (55.3%) instances [57]. All re-directed search results were reviewed 1 month later and all of the unrelated paths had been de-activated.
All websites offered baclofen as either tablets or pills, with the exception of a single website offering powder for research use [58]. None of the websites offered ampoules for intrathecal administration. Baclofen was available as either 10 mg tablets (40 (100%) websites), 20 mg tablets (3 (7.5%)) or 25 mg tablets (29 (72.5%)). The advertised purchase price of baclofen generally declined with increasing volume purchased but was not correlated significantly for either 10 mg (Spearman r = − 0.55; p = 0.10) or 25 mg tablets (r = − 0.65; p = 0.07) (Fig. 2). For websites offering more than one dose, the mean ± SD cost of baclofen was significantly lower for the 10-mg preparation (£54.47/g ± 17.45) than for the 25-mg preparation (£78.81/g ± 23.56; p < 0.01).
Website characteristics
Thirty-eight of the 40 websites advertised ship** of baclofen tablets to the UK, with only a single website operating with appropriate regulatory approval. Further information on website characteristics can be found in Supplementary Data.
Discussion
Since the first case of GHB withdrawal reported in the medical literature in 1994 [7], there has been uncertainty as to the most effective pharmacotherapy to manage this life-threatening condition. High-dose benzodiazepines have become established as the first line approach, probably due to clinician familiarity with their titrated use in alcohol withdrawal. However, as demonstrated in the cases identified, high-dose benzodiazepines are not always sufficient to manage GHB withdrawal [19, 31, 35].
Despite the clinical experience of our centre in which we have successfully treated dozens of patients with GHB/GBL withdrawal with baclofen, there is little published data with regard to the use of baclofen to manage GHB withdrawal. The case reports we identified suggested clinical utility with an acceptable safety profile, but it is not possible to draw definitive conclusions in the absence of randomised controlled trials. Furthermore, despite its described use in both inpatient and outpatient settings, there remains a potential risk of significant harm in baclofen overdose [36, 59].
To augment the limited published literature on the topic, we applied data triangulation techniques on publicly accessible Internet resources to identify sources of ‘grey literature’. We reviewed threads across eight different Internet forums and found multiple mentions of baclofen used to treat GHB withdrawal. The earliest thread identified was published in 2008 and corresponded to the publication of the second case report [19].
The grey literature is at least as extensive as the published scientific literature, with clear caveats relating to accuracy of information and lack of peer review. The dangers of grey literature are clearly demonstrated by one forum post recommending 4 g baclofen to manage withdrawal [47]. The use of baclofen for the management of GHB/GBL withdrawal is off-label, but for other indications, the British National Formulary recommends a starting dose of 5 mg TDS with titration to a maximal daily dose of 100 mg [60]. Doses greater than 200 mg are associated with reduced conscious level, hypotonia and respiratory depression [61], with death having been previously reported after ingestion of 1.25–2.5 g baclofen [59].
The Internet snapshot survey revealed that baclofen is freely available to purchase from online pharmacies that appear to operate contrary to accepted legal and ethical standards. UK/EU registered online pharmacies represent the minority of retailers offering baclofen for sale. It is not possible to determine whether the unregistered online pharmacies are unaware of the legislative requirements or whether they are deliberately contravening the legislation, although a number of factors point towards the latter situation in many instances. The obscuration of both domain ownership and physical location, advertised prescription-free service and the apparent hacking on unrelated websites to generate Internet traffic all suggest deliberate illegal activity. These data are consistent with a report by The Center for Safe Internet Pharmacies which estimates that there are 30–35,000 unregulated online pharmacy websites that are operated by 2–3500 parties [62]. The principle danger associated with these websites is that consumers can self-prescribe from an almost limitless catalogue of medicines without regulatory assurances that these medicines are not counterfeit, unlicensed or withdrawn, and without oversight of the process by a qualified healthcare professional [62, 63].
Despite recent action to suspend UK-based online pharmacies where Care Quality Commission (CQC) inspectors found ‘significant clinical safety and organisational risk to patients’ [64, 65], the current regulatory approach of focusing only on UK-based providers is failing to control online pharmacies whose activities are not restricted by national borders. There is no internationally accepted definition to categorise the illegal selling of medicines, and this combined with the absence of centralised Internet governance has led to fragmented regulation [66]. Until there is a coordinated international approach to both regulation and consumer education, then these websites will continue to put the public at risk of serious harm.
Conclusions
Despite GHB dependency and withdrawal representing a growing public health challenge, there is a lack of robust clinical data to guide its management. Pre-clinical data combined with a limited number of case reports suggest that the GABA-B agonist baclofen is a useful adjunct to high-dose benzodiazepines, and that its use within a supervised clinical setting is safe. However, Internet forums demonstrate that the use of baclofen to treat GHB withdrawal is not limited to healthcare professionals as self-medication using wide variation in dosage is accepted within the online community. Despite baclofen being a POM, our research into online pharmacies has revealed that it is freely available without prescription and that the majority of online pharmacies identified are non-compliant with UK regulation. This work has highlighted that clinical studies are required into the efficacy of baclofen in the treatment of GHB withdrawal, and that current regulation of online pharmacies is inadequate to protect the general public from harm.
Statement on contributorship and the guarantor
CNF collected the primary data and undertook the initial data analysis; all authors contributed equally to the drafting of this manuscript. PID acts as guarantor.
References
Laborit H (1964) Sodium 4-hydroxybutyrate. Int J Neuropharmacol 3(4):433–451. https://doi.org/10.1016/0028-3908(64)90074-7
Busardo FP et al (2015) Clinical applications of sodium oxybate (GHB): from narcolepsy to alcohol withdrawal syndrome. Eur Rev Med Pharmacol Sci 19(23):4654–4663
Takahara J, Yunoki S, Yakushiji W, Yamauchi J, Yamane Y, Ofuji T (1977) Stimulatory effects of gamma-hydroxybutyric acid on growth hormone and prolactin release in humans. J Clin Endocrinol Metab 44(5):1014–1017. https://doi.org/10.1210/jcem-44-5-1014
Degenhardt L, Darke S, Dillon P (2002) GHB use among Australians: characteristics, use patterns and associated harm. Drug Alcohol Depend 67(1):89–94. https://doi.org/10.1016/S0376-8716(02)00017-0
Felmlee MA, Roiko SA, Morse BL, Morris ME (2010) Concentration-effect relationships for the drug of abuse gamma-hydroxybutyric acid. J Pharmacol Exp Ther 333(3):764–771. https://doi.org/10.1124/jpet.109.165381
Wood DM, Brailsford AD, Dargan PI (2011) Acute toxicity and withdrawal syndromes related to gamma-hydroxybutyrate (GHB) and its analogues gamma-butyrolactone (GBL) and 1,4-butanediol (1,4-BD). Drug Test Anal 3(7–8):417–425. https://doi.org/10.1002/dta.292
Galloway GP, Frederick SL, Staggers F Jr (1994) Physical dependence on sodium oxybate. Lancet 343(8888):57. https://doi.org/10.1016/S0140-6736(94)90911-3
Addolorato G, Caputo F, Stefanini GF, Gasbarrini G (1997) Gamma-hydroxybutyric acid in the treatment of alcohol dependence: possible craving development for the drug. Addiction 92(8):1035–1036. https://doi.org/10.1111/j.1360-0443.1997.tb02984.x
Galloway GP et al (1997) Gamma-hydroxybutyrate: an emerging drug of abuse that causes physical dependence. Addiction 92(1):89–96. https://doi.org/10.1111/j.1360-0443.1997.tb03640.x
Davies M (2003) The role of GABAA receptors in mediating the effects of alcohol in the central nervous system. J Psychiatry Neurosci 28(4):263–274
Liechti ME, Quednow BB, Liakoni E, Dornbierer D, von Rotz R, Gachet MS, Gertsch J, Seifritz E, Bosch OG (2016) Pharmacokinetics and pharmacodynamics of gamma-hydroxybutyrate in healthy subjects. Br J Clin Pharmacol 81(5):980–988. https://doi.org/10.1111/bcp.12863
Choudhuri D, Cross S, Dargan PI, Wood DM, Ranjith G (2013) Psychiatric aspects of acute withdrawal from gamma-hydroxybutyrate (GHB) and its analogue gamma-butyrolactone (GBL): implications for psychiatry services in the general hospital. Int J Psychiatry Clin Pract 17(2):154–156. https://doi.org/10.3109/13651501.2013.784790
Kamal RM, van Noorden MS, Wannet W, Beurmanjer H, Dijkstra BAG, Schellekens A (2017) Pharmacological treatment in gamma-hydroxybutyrate (GHB) and gamma-butyrolactone (GBL) dependence: detoxification and relapse prevention. CNS Drugs 31(1):51–64. https://doi.org/10.1007/s40263-016-0402-z
Ong J, Kerr DI (2005) Clinical potential of GABAB receptor modulators. CNS Drug Rev 11(3):317–334
Hill DR, Bowery NG (1981) 3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain. Nature 290(5802):149–152. https://doi.org/10.1038/290149a0
Milanov IG (1992) Mechanisms of baclofen action on spasticity. Acta Neurol Scand 85(5):305–310
Goodwin AK, Froestl W, Weerts EM (2005) Involvement of gamma-hydroxybutyrate (GHB) and GABA-B receptors in the acute behavioral effects of GHB in baboons. Psychopharmacology 180(2):342–351. https://doi.org/10.1007/s00213-005-2165-y
Smith MA, Gergans SR, Lyle MA (2006) The motor-impairing effects of GABA(A) and GABA(B) agonists in gamma-hydroxybutyrate (GHB)-treated rats: cross-tolerance to baclofen but not flunitrazepam. Eur J Pharmacol 552(1–3):83–89. https://doi.org/10.1016/j.ejphar.2006.08.080
LeTourneau JL, Hagg DS, Smith SM (2008) Baclofen and gamma-hydroxybutyrate withdrawal. Neurocrit Care 8(3):430–433. https://doi.org/10.1007/s12028-008-9062-2
Ho JH, Bailey GP, Archer JRH, Dargan PI, Wood DM (2015) Ethylphenidate: availability, patterns of use, and acute effects of this novel psychoactive substance. Eur J Clin Pharmacol 71(10):1185–1196. https://doi.org/10.1007/s00228-015-1906-z
Hillebrand J, Olszewski D, Sedefov R (2010) Legal highs on the Internet. Subst Use Misuse 45(3):330–340. https://doi.org/10.3109/10826080903443628
European Commission. INFOREURO Currency Converter. 6th March 2017]; Available from: http://ec.europa.eu/budget/contracts_grants/info_contracts/inforeuro/index_en.cfm
Daigle L. WHOIS Protocol Specification. . 2004 4th May 2017]; Available from: https://tools.ietf.org/html/rfc3912
Medicines and Healthcare products Regulatory Agency. 05 May 2017]; Available from: https://medicine-seller-register.mhra.gov.uk/
General Pharmaceutical Council. 6th July 2017]; Available from: https://www.pharmacyregulation.org/registration/internet-pharmacy
Secretary’s Advisory Committee on Human Research Protections (SACHRP). Considerations and recommendations concerning Internet research and human subjects research regulations, with revisions. . 2013 6th July 2017]; Available from: https://www.hhs.gov/ohrp/sites/default/files/ohrp/sachrp/mtgings/2013%20March%20Mtg/internet_research.pdf
Bell J, Collins R (2011) Gamma-butyrolactone (GBL) dependence and withdrawal. Addiction 106(2):442–447. https://doi.org/10.1111/j.1360-0443.2010.03145.x
Kamal RM, Loonen AJM, Dijkstra BAG, de Jong CAJ (2015) Baclofen as relapse prevention in the treatment of gamma-hydroxybutyrate dependence: a case series. J Clin Psychopharmacol 35(3):313–318. https://doi.org/10.1097/JCP.0000000000000315
Kamal RM, Schellekens A, de Jong CAJ, Dijkstra BAG (2015) Baclofen as relapse prevention in the treatment of gamma- hydroxybutyrate (GHB) dependence: an open label study. BMC Psychiatry 15(1):91. https://doi.org/10.1186/s12888-015-0471-4
Lingford-Hughes A, Patel Y, Bowden-Jones O, Crawford MJ, Dargan PI, Gordon F, Parrott S, Weaver T, Wood DM (2016) Improving GHB withdrawal with baclofen: study protocol for a feasibility study for a randomised controlled trial. Trials 17(1):472. https://doi.org/10.1186/s13063-016-1593-9
Acciani J, Kao L (2010) Severe GHB withdrawal treated with baclofen. Clin Toxicol 48(6):617
Colby DK et al (2015) 1,4 butanediol withdrawal and pharmacologic management: a case series. Clin Toxicol 53(7):675
McDonough M (2013) GHB dependence: lessons from a small case series. J Alcoholism Drug Depend 1:112
Wood D, et al. Management of acute gamma-hydroxybutyrate (GHB) and gamma-butyrolactone (GBL) withdrawal: use of CIWA-Ar scoring is not sufficient in determining need for benzodiazepine treatment. 2011 6th July 2017]; Available from: https://bps.conference-services.net/resources/344/2833/pdf/BPSWINTER2011_0152.pdf
McDaniel CH, Miotto KA (2001) Gamma hydroxybutyrate (GHB) and gamma butyrolactone (GBL) withdrawal: five case studies. J Psychoactive Drugs 33(2):143–149. https://doi.org/10.1080/02791072.2001.10400479
Kamal RM, Qurishi R, De Jong CA (2015) Baclofen and gamma-hydroxybutyrate (GHB), a dangerous combination. J Addict Med 9(1):75–77. https://doi.org/10.1097/ADM.0000000000000084
Bluelight. 7th July 2017]; Available from: http://www.bluelight.org/vb/content/
My Way Out. 7th July 2017]; Available from: http://www.MyWayOut.org
Drugs-Forum. 7th July 2017]; Available from: http://Drugs-forum.com
Social Anxiety Support. 7th July 2017]; Available from: http://www.socialanxietysupport.com
Mind and Muscle Bodybuilding. 7th July 2017]; Available from: http://forum.mindandmuscle.net
LongeCity. 7th July 2017]; Available from: http://www.longecity.org
Dialogue Space. 7th July 2017]; Available from: http://www.dialogue.space/
No More Panic. 7th July 2017]; Available from: No More Panic
Drugs-Forum. Alcohol Abuse and G. 2008 7th July 2017]; Available from: https://drugs-forum.com/threads/alcohol-abuse-and-g.56912/
Dialogue Space. GBL withdrawel. 7th July 2017]; Available from: http://www.dialogue.space/gbl-withdrawel/
dialogue Space. GBL urgent help needed. 7th July 2017]; Available from: http://www.dialogue.space/gbl-urgent-help-needed/
Drugs-Forum. Benzos, GBL; A Novel Withdrawal Process...Draft. 7th July 2017]; Available from: https://drugs-forum.com/threads/benzos-gbl-a-novel-withdrawal-process-draft.80584/
Bluelight. GBL/ GHB withdrawal HELP. 7th July 2017]; Available from: http://www.bluelight.org/vb/threads/328200-GBL-GHB-withdrawal-HELP?p=13787483&viewfull=1#post13787483
Drugs-Forum. Guidance for managment of GBL and 1,4-BD withdrawal and detox. Available from: https://drugs-forum.com/threads/guidance-for-managment-of-gbl-and-1-4-bd-withdrawal-and-detox.172924
Taylor D, Paton C, and Kapur S. The Maudsley prescribing guidelines in psychiatry. 2015 7th July 2017]; Available from: https://books.google.co.uk/books?id=Lb77CAAAQBAJ&pg=PA468&lpg=PA468&dq=baclofen+gbl+withdrawal&source=bl&ots=__9qtxIjOJ&sig=kXo2Czt9KC6djQ6Gq9ba5O3FivQ&hl=en&sa=X&ved=0ahUKEwia07irluXSAhWBKcAKHdPiDNQ4FBDoAQgsMAM#v=onepage&q=baclofen%20gbl%20withdrawal&f=false
Northwest Addiction Specialists. Manchester GHB information pack (Final).doc. 7th July 2017]; Available from: http://www.google.co.uk/url?sa=t&rct=j&q=&esrc=s&source=web&cd=10&ved=0ahUKEwiPqay6leXSAhUpAsAKHchPAG4QFghjMAk&url=http%3A%2F%2Fwww.dualdiagnosis.co.uk%2Fuploads%2Fdocuments%2Foriginals%2FManchester%2520GHB%2520information%2520pack%2520%28Final%29.doc&usg=AFQjCNHIdgkJIfy-cx8WIKY6JtA4OIKGNA
Private Drug Rehab Center. 7th July 2017]; Available from: http://www.private-drug-rehab-center.com/ghb-drug-facts.php
Creative Care. 7th July 2017]; Available from: http://www.creativecareinc.com/ghb-addiction-treatment/
Addiction Search. 7th July 2017]; Available from: http://www.addictionsearch.com/treatment_articles/article/ghb-addiction-abuse-detox-and-treatment_27.html
Club Drug Clinic. 7th July 2017]; Available from: http://clubdrugclinic.cnwl.nhs.uk/
Pharmacy Shop 24/7. 7th Febrary 2017]; Available from: http://pharmacyshop24x7.com/product/Baclofen.html?q=Baclofen
Selleckchem.com. 7th July 2017]; Available from: www.selleckchem.com/products/r-baclofen.html
Haubenstock A, Hruby K, Jäger U, Lenz K (1983) Baclofen (Lioresal) intoxication report of 4 cases and review of the literature. J Toxicol Clin Toxicol 20(1):59–68. https://doi.org/10.3109/15563658308990050
British National Formulary. Baclofen. 13th March 2017]; Available from: https://www.evidence.nhs.uk/formulary/bnf/current/10-musculoskeletal-and-joint-diseases/102-drugs-used-in-neuromuscular-disorders/1022-skeletal-muscle-relaxants/baclofen
Leung NY, Whyte IM, Isbister GK (2006) Baclofen overdose: defining the spectrum of toxicity. Emerg Med Australas 18(1):77–82. https://doi.org/10.1111/j.1742-6723.2006.00805.x
The Center for Safe Internet Pharmacies. The Internet Pharmacy Market in 2016. 3rd May 2017]; Available from: http://www.safemedsonline.org/wp-content/uploads/2016/01/The-Internet-Pharmacy-Market-in-2016.pdf
Orizio G, Schulz P, Domenighini S, Caimi L, Rosati C, Rubinelli S, Gelatti U (2009) Cyberdrugs: a cross-sectional study of online pharmacies characteristics. Eur J Pub Health 19(4):375–377. https://doi.org/10.1093/eurpub/ckn146
Care Quality Commission. Inspection Report: MD Direct Ltd. . 3 March 2017 7th March 2017]; Available from: http://www.cqc.org.uk/sites/default/files/new_reports/AAAG0839.pdf
Care Quality Commission. Inspection Report: HR Healthcare Ltd. . 3 March 2017 7th March 2017]; Available from: http://www.cqc.org.uk/sites/default/files/new_reports/AAAG0834.pdf
Anderson AC, Mackey TK, Attaran A, Liang BA (2016) Map** of health communication and education strategies addressing the public health dangers of illicit online pharmacies. J Health Commun 21(4):397–407. https://doi.org/10.1080/10810730.2015.1095816
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
This research was approved by the King’s College London Research Ethics Committee (reference RESCMR-16/17-2097).
Competing interests
We have read and understood the EJCP policy on declaration of interests and declare the following interests:
PID is a member of the UK Advisory Council on the Misuse of Drugs, the European Monitoring Centre for Drugs and Drug Addiction Scientific Committee and the Glaxo Smith Kline Global Analgesics Advisory Panel and an expert advisor to the World Health Organisation and United Nations Office on Drugs and Crime.
DMW is expert advisor to the UK Advisory Council on the Misuse of Drugs, the European Monitoring Centre for Drugs and Drug Addiction and the United Nations Office on Drugs and Crime.
Electronic supplementary material
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Floyd, C.N., Wood, D.M. & Dargan, P.I. Baclofen in gamma-hydroxybutyrate withdrawal: patterns of use and online availability. Eur J Clin Pharmacol 74, 349–356 (2018). https://doi.org/10.1007/s00228-017-2387-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-017-2387-z