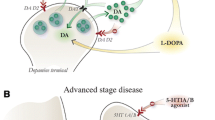
Abstract
The understanding of Parkinson’s disease (PD) classically revolves around dopamine depletion within the striatum. However, PD is a multi-systemic disease in which extra-dopaminergic systems are affected. The serotonergic (5-HT) system is one of these and has been extensively studied in PD. Although the 5-HT system uses one transporter (SERT) and 14 receptor sub-types, most of the studies in PD have focussed on SERT and serotonergic type 1A and 2A receptors (5-HT1A and 5-HT2A). Post-mortem autoradiographic binding studies and in vivo imaging studies have suggested an involvement of the 5-HT system in PD-related anxiety, depression, psychosis and L-3,4-dihydroxyphenylalanine (L-DOPA)-induced dyskinesia. Pre-clinical and clinical pharmacological studies have shown that SERT blockade might effectively alleviate depression and dyskinesia and, more recently, might exert disease-modifying effects. Enhancing the physiological activity of 5-HT1A receptors with 5-HT1A agonists might alleviate anxiety, dyskinesia and tremor, although a deleterious effect on the anti-parkinsonian efficacy of L-DOPA may ultimately limit the use of this class of compounds. Enhanced 5-HT2A-mediated neurotransmission has been associated with depression, dyskinesia, psychosis and tremor. The current article critically reviews studies assessing the SERT, as well as 5-HT1A and 5-HT2A receptors in idiopathic PD and animal models of PD, and discusses unmet challenges to effectively treat manifestations of PD using SERT antagonists, 5-HT1A agonists and 5-HT2A antagonists.
Similar content being viewed by others
References
ACADIA (2013) ACADIA Pharmaceuticals announces expedited path to NDA filing for pimavanserin following meeting with FDA. http://phx.corporate-ir.net/phoenix.zhtml?c=125180&p=irol-newsArticle&ID=1805706 Accessed 14th April 2013
Albin RL, Koeppe RA, Bohnen NI, Wernette K, Kilbourn MA, Frey KA (2008) Spared caudal brainstem SERT binding in early Parkinson’s disease. J Cereb Blood Flow Metab 28:441–444
Antonini A, Tesei S, Zecchinelli A et al (2006) Randomized study of sertraline and low-dose amitriptyline in patients with Parkinson’s disease and depression: effect on quality of life. Mov Disord 21:1119–1122
Arai R, Karasawa N, Geffard M, Nagatsu T, Nagatsu I (1994) Immunohistochemical evidence that central serotonin neurons produce dopamine from exogenous L-DOPA in the rat, with reference to the involvement of aromatic L-amino acid decarboxylase. Brain Res 667:295–299
Arai R, Karasawa N, Geffard M, Nagatsu I (1995) L-DOPA is converted to dopamine in serotonergic fibers of the striatum of the rat: a double-labeling immunofluorescence study. Neurosci Lett 195:195–198
Arai R, Karasawa N, Nagatsu I (1996) Aromatic L-amino acid decarboxylase is present in serotonergic fibers of the striatum of the rat. A double-labeling immunofluorescence study. Brain Res 706:177–179
Auff E, Birkmayer W, Brucke T et al (1987) Ritanserin in the treatment of tremor-dominant Parkinson’s disease: a preliminary study. New Trends Clin Neuropharmacol 1:149–158
Ballanger B, Strafella AP, van Eimeren T, Zurowski M, Rusjan PM, Houle S, Fox SH (2010) Serotonin 2A receptors and visual hallucinations in Parkinson disease. Arch Neurol 67:416–421
Ballanger B, Klinger H, Eche J et al (2012) Role of serotonergic 1A receptor dysfunction in depression associated with Parkinson’s disease. Mov Disord 27:84–89
Bara-Jimenez W, Bibbiani F, Morris MJ, Dimitrova T, Sherzai A, Mouradian MM, Chase TN (2005) Effects of serotonin 5-HT1A agonist in advanced Parkinson’s disease. Mov Disord 20:932–936
Barnes NM, Sharp T (1999) A review of central 5-HT receptors and their function. Neuropharmacology 38:1083–1152
Baron MS, Dalton WB (2003) Quetiapine as treatment for dopaminergic-induced dyskinesias in Parkinson’s disease. Mov Disord 18:1208–1209
Bartoszyk GD, Van den Buuse M, Gerlach M, Riederer P (2006) Mechanism of the antidyskinetic efficacy of sarizotan in hemiparkinsonian rats [Abstract]. Mov Disord 21(Suppl 15):S495
Bennett JP Jr, Landow ER, Schuh LA (1993) Suppression of dyskinesias in advanced Parkinson’s disease. II. Increasing daily clozapine doses suppress dyskinesias and improve parkinsonism symptoms. Neurology 43:1551–1555
Bennett JP Jr, Landow ER, Dietrich S, Schuh LA (1994) Suppression of dyskinesias in advanced Parkinson’s disease: moderate daily clozapine doses provide long-term dyskinesia reduction. Mov Disord 9:409–414
Berding G, Brucke T, Odin P et al (2003) [[123I]beta-CIT SPECT imaging of dopamine and serotonin transporters in Parkinson’s disease and multiple system atrophy. Nuklearmedizin 42:31–38
Berger B (1978) In vitro uptake of dopamine in serotoninergic nerve terminals: a fluorescence histochemical study on vibratome sections of the rat cerebral cortex. Adv Biochem Psychopharmacol 19:405–408
Berger B, Glowinski J (1978) Dopamine uptake in serotoninergic terminals in vitro: a valuable tool for the histochemical differentiation of catecholaminergic and serotoninergic terminals in rat cerebral structures. Brain Res 147:29–45
Bibbiani F, Oh JD, Chase TN (2001) Serotonin 5-HT1A agonist improves motor complications in rodent and primate parkinsonian models. Neurology 57:1829–1834
Bishop C, Taylor JL, Kuhn DM, Eskow KL, Park JY, Walker PD (2006) MDMA and fenfluramine reduce L-DOPA-induced dyskinesia via indirect 5-HT1A receptor stimulation. Eur J Neurosci 23:2669–2676
Bishop C, Krolewski DM, Eskow KL, Barnum CJ, Dupre KB, Deak T, Walker PD (2009) Contribution of the striatum to the effects of 5-HT1A receptor stimulation in L-DOPA-treated hemiparkinsonian rats. J Neurosci Res 87:1645–1658
Bishop C, George JA, Buchta W et al (2012) Serotonin transporter inhibition attenuates l-DOPA-induced dyskinesia without compromising l-DOPA efficacy in hemi-parkinsonian rats. Eur J Neurosci 36:2839–2848
Boileau I, Warsh JJ, Guttman M et al (2008) Elevated serotonin transporter binding in depressed patients with Parkinson’s disease: a preliminary PET study with [11C]DASB. Mov Disord 23:1776–1780
Bonhaus D, MacFarland K, Willims H, Mills R (2009) Pimavanserin: a selective 5-HT2A inverse agonist has antipsychotic like activity in animal models of Parkinson’s disease (PD) and reduces psychotic symptoms in PD patients [Abstract]. Soc Neurosci
Bonifati V, Fabrizio E, Cipriani R, Vanacore N, Meco G (1994) Buspirone in levodopa-induced dyskinesias. Clin Neuropharmacol 17:73–82
Boyer EW, Shannon M (2005) The serotonin syndrome. N Engl J Med 352:1112–1120
Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E (2003) Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol Aging 24:197–211
Braak H, Ghebremedhin E, Rub U, Bratzke H, Del Tredici K (2004) Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res 318:121–134
Brandstadter D, Oertel WH (2002) Treatment of drug-induced psychosis with quetiapine and clozapine in Parkinson’s disease. Neurology 58:160–161
Brucke T, Kornhuber J, Angelberger P, Asenbaum S, Frassine H, Podreka I (1993) SPECT imaging of dopamine and serotonin transporters with [123I]beta-CIT. Binding kinetics in the human brain. J Neural Transm Gen Sect 94:137–146
Burstein ES, Ma J, Wong S et al (2005) Intrinsic efficacy of antipsychotics at human D2, D3, and D4 dopamine receptors: identification of the clozapine metabolite N-desmethylclozapine as a D2/D3 partial agonist. J Pharmacol Exp Ther 315:1278–1287
Bymaster FP, Calligaro DO, Falcone JF et al (1996) Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology 14:87–96
Caley CF, Friedman JH (1992) Does fluoxetine exacerbate Parkinson’s disease? J Clin Psychiatry 53:278–282
Caretti V, Stoffers D, Winogrodzka A et al (2008) Loss of thalamic serotonin transporters in early drug-naive Parkinson’s disease patients is associated with tremor: an [(123)I]beta-CIT SPECT study. J Neural Transm 115:721–729
Carlsson T, Carta M, Winkler C, Bjorklund A, Kirik D (2007) Serotonin neuron transplants exacerbate L-DOPA-induced dyskinesias in a rat model of Parkinson’s disease. J Neurosci 27:8011–8022
Carlsson T, Carta M, Munoz A, Mattsson B, Winkler C, Kirik D, Bjorklund A (2009) Impact of grafted serotonin and dopamine neurons on development of L-DOPA-induced dyskinesias in parkinsonian rats is determined by the extent of dopamine neuron degeneration. Brain 132:319–335
Carta M, Carlsson T, Kirik D, Bjorklund A (2007) Dopamine released from 5-HT terminals is the cause of L-DOPA-induced dyskinesia in parkinsonian rats. Brain 130:1819–1833
Carta M, Carlsson T, Munoz A, Kirik D, Bjorklund A (2008) Serotonin-dopamine interaction in the induction and maintenance of L-DOPA-induced dyskinesias. Prog Brain Res 172:465–478
Ceravolo R, Nuti A, Piccinni A et al (2000) Paroxetine in Parkinson’s disease: effects on motor and depressive symptoms. Neurology 55:1216–1218
Chen CP, Alder JT, Bray L, Kingsbury AE, Francis PT, Foster OJ (1998) Post-synaptic 5-HT1A and 5-HT2A receptors are increased in Parkinson’s disease neocortex. Ann N Y Acad Sci 861:288–289
Chen P, Kales HC, Weintraub D, Blow FC, Jiang L, Mellow AM (2007) Antidepressant treatment of veterans with Parkinson’s disease and depression: analysis of a national sample. J Geriatr Psychiatry Neurol 20:161–165
Cheng AV, Ferrier IN, Morris CM et al (1991) Cortical serotonin-S2 receptor binding in Lewy body dementia, Alzheimer’s and Parkinson’s diseases. J Neurol Sci 106:50–55
Chinaglia G, Landwehrmeyer B, Probst A, Palacios JM (1993) Serotoninergic terminal transporters are differentially affected in Parkinson’s disease and progressive supranuclear palsy: an autoradiographic study with [3H]citalopram. Neuroscience 54:691–699
Chung YC, Kim SR, Park JY et al (2011) Fluoxetine prevents MPTP-induced loss of dopaminergic neurons by inhibiting microglial activation. Neuropharmacology 60:963–974
Connemann BJ, Schonfeldt-Lecuona C (2004) Ziprasidone in Parkinson’s disease psychosis. Can J Psychiatry 49:73
Dahlstroem A, Fuxe K (1964) Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons. Acta Physiol Scand 232(Suppl):231–255
D’Amato RJ, Zweig RM, Whitehouse PJ et al (1987) Aminergic systems in Alzheimer’s disease and Parkinson’s disease. Ann Neurol 22:229–236
Dekundy A, Lundblad M, Danysz W, Cenci MA (2007) Modulation of L-DOPA-induced abnormal involuntary movements by clinically tested compounds: further validation of the rat dyskinesia model. Behav Brain Res 179:76–89
Descarries L, Riad M (2012) Effects of the antidepressant fluoxetine on the subcellular localization of 5-HT1A receptors and SERT. Philos Trans R Soc Lond B Biol Sci 367:2416–2425
Dewey RB Jr, O’Suilleabhain PE (2000) Treatment of drug-induced psychosis with quetiapine and clozapine in Parkinson’s disease. Neurology 55:1753–1754
Doder M, Rabiner EA, Turjanski N, Lees AJ, Brooks DJ (2003) Tremor in Parkinson’s disease and serotonergic dysfunction: an 11C-WAY 100635 PET study. Neurology 60:601–605
Dupre KB, Eskow KL, Negron G, Bishop C (2007) The differential effects of 5-HT(1A) receptor stimulation on dopamine receptor-mediated abnormal involuntary movements and rotations in the primed hemiparkinsonian rat. Brain Res 1158:135–143
Dupre KB, Eskow KL, Barnum CJ, Bishop C (2008) Striatal 5-HT1A receptor stimulation reduces D1 receptor-induced dyskinesia and improves movement in the hemiparkinsonian rat. Neuropharmacology 55:1321–1328
Dupre KB, Ostock CY, Eskow Jaunarajs KL, Button T, Savage LM, Wolf W, Bishop C (2011) Local modulation of striatal glutamate efflux by serotonin 1A receptor stimulation in dyskinetic, hemiparkinsonian rats. Exp Neurol 229:288–299
Durif F, Vidailhet M, Bonnet AM, Blin J, Agid Y (1995) Levodopa-induced dyskinesias are improved by fluoxetine. Neurology 45:1855–1858
Eskow KL, Gupta V, Alam S, Park JY, Bishop C (2007) The partial 5-HT(1A) agonist buspirone reduces the expression and development of l-DOPA-induced dyskinesia in rats and improves l-DOPA efficacy. Pharmacol Biochem Behav 87:306–314
Eskow KL, Dupre KB, Barnum CJ, Dickinson SO, Park JY, Bishop C (2009) The role of the dorsal raphe nucleus in the development, expression, and treatment of L-DOPA-induced dyskinesia in hemiparkinsonian rats. Synapse 63:610–620
Factor SA, Brown D (1992) Clozapine prevents recurrence of psychosis in Parkinson’s disease. Mov Disord 7:125–131
Factor SA, Friedman JH (1997) The emerging role of clozapine in the treatment of movement disorders. Mov Disord 12:483–496
Fahn S (2008) The history of dopamine and levodopa in the treatment of Parkinson’s disease. Mov Disord 23(Suppl 3):S497–S508
Fahn S, Libsch LR, Cutler RW (1971) Monoamines in the human neostriatum: topographic distribution in normals and in Parkinson’s disease and their role in akinesia, rigidity, chorea, and tremor. J Neurol Sci 14:427–455
Fahn S, Elton RL, Members of the UPDRS Development Committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Goldstein M, Calne DB (eds) Recent developments in Parkinson’s disease. Macmillan Healthcare Information, Florham Park, pp 153–163
Fernandez HH, Trieschmann ME, Burke MA, Jacques C, Friedman JH (2003) Long-term outcome of quetiapine use for psychosis among Parkinsonian patients. Mov Disord 18:510–514
Fernandez HH, Trieschmann ME, Friedman JH (2004) Aripiprazole for drug-induced psychosis in Parkinson disease: preliminary experience. Clin Neuropharmacol 27:4–5
Fox SH, Visanji N, Reyes G, Huot P, Gomez-Ramirez J, Johnston T, Brotchie JM (2010) Neuropsychiatric behaviors in the MPTP marmoset model of Parkinson’s disease. Can J Neurol Sci 37:86–95
Fregni F, Santos CM, Myczkowski ML et al (2004) Repetitive transcranial magnetic stimulation is as effective as fluoxetine in the treatment of depression in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 75:1171–1174
French Clozapine Parkinson Study Group (1999) Clozapine in drug-induced psychosis in Parkinson’s disease. The French Clozapine Parkinson Study Group. Lancet 353:2041–2042
Friedman JH (1992) Fluoxetine in Parkinson’s disease [Abstract]. Mov Disord 7(Suppl 1):102
Friedman JH, Factor SA (2000) Atypical antipsychotics in the treatment of drug-induced psychosis in Parkinson’s disease. Mov Disord 15:201–211
Friedman JH, Lannon MC (1989) Clozapine in the treatment of psychosis in Parkinson’s disease. Neurology 39:1219–1221
Friedman JH, Berman RM, Goetz CG et al (2006) Open-label flexible-dose pilot study to evaluate the safety and tolerability of aripiprazole in patients with psychosis associated with Parkinson’s disease. Mov Disord 21:2078–2081
Frisina PG, Haroutunian V, Libow LS (2009) The neuropathological basis for depression in Parkinson’s disease. Parkinsonism Relat Disord 15:144–148
Gatto EM, Fernandez Pardal M, Micheli F (1994) Exacerbation of parkinsonism caused by fluoxetine. Medicina (B Aires) 54:182
Gerard C, Martres MP, Lefevre K et al (1997) Immuno-localization of serotonin 5-HT6 receptor-like material in the rat central nervous system. Brain Res 746:207–219
Gil S, Park C, Lee J, Koh H (2010) The roles of striatal serotonin and L: -amino-acid decarboxylase on L: -DOPA-induced Dyskinesia in a Hemiparkinsonian rat model. Cell Mol Neurobiol 30:817–825
Gil SJ, Park CH, Lee JE, Minn YK, Koh HC (2011) Positive association between striatal serotonin level and abnormal involuntary movements in chronic L-DOPA-treated hemiparkinsonian rats. Brain Res Bull 84:151–156
Gimenez-Roldan S, Navarro E, Mateo D (2003) Effects of quetiapine at low doses on psychosis motor disability and stress of the caregiver in patients with Parkinson’s disease. Rev Neurol 36:401–404
Goetz CG, Damier P, Hicking C et al (2007) Sarizotan as a treatment for dyskinesias in Parkinson’s disease: a double-blind placebo-controlled trial. Mov Disord 22:179–186
Gomez-Mancilla B, Bedard PJ (1993) Effect of nondopaminergic drugs on L-DOPA-induced dyskinesias in MPTP-treated monkeys. Clin Neuropharmacol 16:418–427
Gordon PH, Pullman SL, Louis ED, Frucht SJ, Fahn S (2002) Mirtazapine in Parkinsonian tremor. Parkinsonism Relat Disord 9:125–126
Gregoire L, Samadi P, Graham J, Bedard PJ, Bartoszyk GD, Di Paolo T (2009) Low doses of sarizotan reduce dyskinesias and maintain antiparkinsonian efficacy of L-DOPA in parkinsonian monkeys. Parkinsonism Relat Disord 15:445–452
Guttman M, Boileau I, Warsh J et al (2007) Brain serotonin transporter binding in non-depressed patients with Parkinson’s disease. Eur J Neurol 14:523–528
Haapaniemi TH, Ahonen A, Torniainen P, Sotaniemi KA, Myllyla VV (2001) [123I]beta-CIT SPECT demonstrates decreased brain dopamine and serotonin transporter levels in untreated parkinsonian patients. Mov Disord 16:124–130
Halliday GM, Blumbergs PC, Cotton RG, Blessing WW, Geffen LB (1990a) Loss of brainstem serotonin- and substance P-containing neurons in Parkinson’s disease. Brain Res 510:104–107
Halliday GM, Li YW, Blumbergs PC et al (1990b) Neuropathology of immunohistochemically identified brainstem neurons in Parkinson’s disease. Ann Neurol 27:373–385
Hammerstad JP, Carter J, Nutt JG, Casten GC, Shrotriya RC, Alms DR, Temple D (1986) Buspirone in Parkinson’s disease. Clin Neuropharmacol 9:556–560
Hauser RA, Zesiewicz TA (1997) Sertraline for the treatment of depression in Parkinson’s disease. Mov Disord 12:756–759
Hely MA, Morris JG, Reid WG, Trafficante R (2005) Sydney Multicenter Study of Parkinson’s disease: non-L-DOPA-responsive problems dominate at 15 years. Mov Disord 20:190–199
Henderson J, Yiannikas C, Graham JS (1992) Effect of ritanserin, a highly selective 5-HT2 receptor antagonist, on Parkinson’s disease. Clin Exp Neurol 29:277–282
Hildebrand J, Delecluse F (1987) Effect of ritanserin, a selective serotonin-S2 antagonist, on parkinsonian rest tremor. Curr Ther Res 41:298–300
Hoglinger GU, Rizk P, Muriel MP, Duyckaerts C, Oertel WH, Caille I, Hirsch EC (2004) Dopamine depletion impairs precursor cell proliferation in Parkinson disease. Nat Neurosci 7:726–735
Hornykiewicz O (2002) L-DOPA: from a biologically inactive amino acid to a successful therapeutic agent. Amino Acids 23:65–70
Hornykiewicz O, Kish SJ (1987) Biochemical pathophysiology of Parkinson’s disease. Adv Neurol 45:19–34
http://www.clinicaltrials.gov/ (NCT01174004) A study of the safety and efficacy of pimavanserin in patients with Parkinson’s disease psychosis. Accessed 14th April 2013
Huot P, Johnston TH, Darr T et al (2010a) Increased 5-HT2A receptors in the temporal cortex of parkinsonian patients with visual hallucinations. Mov Disord 25:1399–1408
Huot P, Johnston TH, Koprich JB, Winkelmolen L, Fox SH, Brotchie JM (2010b) Regulation of cortical and striatal 5-HT(1A) receptors in the MPTP-lesioned macaque. Neurobiol Aging 1:491–512. doi:10.1016/j.neurobiolaging.2010.1009.1011
Huot P, Fox SH, Brotchie JM (2011a) The serotonergic system in Parkinson’s disease. Prog Neurobiol 95:163–212
Huot P, Fox SH, Newman-Tancredi A, Brotchie JM (2011b) Anatomically-selective 5-HT1A and 5-HT2A therapies for Parkinson’s disease—an approach to reducing dyskinesia without exacerbating parkinsonism? J Pharmacol Exp Ther 339:1–7
Huot P, Johnston TH, Lewis KD et al (2011c) Characterization of 3,4-methylenedioxymethamphetamine (mdma) enantiomers in vitro and in the mptp-lesioned primate: R-MDMA reduces severity of dyskinesia, whereas S-MDMA extends duration of on-time. J Neurosci 31:7190–7198
Huot P, Johnston TH, Visanji NP et al (2012a) Increased levels of 5-HT1A receptor binding in ventral visual pathways in Parkinson’s disease. Mov Disord 27:735–742
Huot P, Johnston TH, Winkelmolen L, Fox SH, Brotchie JM (2012b) 5-HT(2A) receptor levels increase in MPTP-lesioned macaques treated chronically with L-DOPA. Neurobiol Aging 33:194.e195-115
Iravani MM, Jackson MJ, Kuoppamaki M, Smith LA, Jenner P (2003) 3,4-methylenedioxymethamphetamine (ecstasy) inhibits dyskinesia expression and normalizes motor activity in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates. J Neurosci 23:9107–9115
Iravani MM, Tayarani-Binazir K, Chu WB, Jackson MJ, Jenner P (2006) In 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-treated primates, the selective 5-hydroxytryptamine 1a agonist (R)-(+)-8-OHDPAT inhibits levodopa-induced dyskinesia but only with increased motor disability. J Pharmacol Exp Ther 319:1225–1234
Jakeman LB, To ZP, Eglen RM, Wong EH, Bonhaus DW (1994) Quantitative autoradiography of 5-HT4 receptors in brains of three species using two structurally distinct radioligands, [3H]GR113808 and [3H]BIMU-1. Neuropharmacology 33:1027–1038
Jordan S, Koprivica V, Chen R, Tottori K, Kikuchi T, Altar CA (2002) The antipsychotic aripiprazole is a potent, partial agonist at the human 5-HT1A receptor. Eur J Pharmacol 441:137–140
Juncos JL, Arvanitis L, Sweitzer D, Yeung P, Jewart RD (1999) Quetiapine improves psychotic symptoms associated with Parkinson’s disease. Neurology 52(Suppl 2):A262
Kannari K, Kurahashi K, Tomiyama M et al (2002) Tandospirone citrate, a selective 5-HT1A agonist, alleviates L-DOPA-induced dyskinesia in patients with Parkinson’s disease. No To Shinkei 54:133–137
Katzenschlager R, Manson AJ, Evans A, Watt H, Lees AJ (2004) Low dose quetiapine for drug induced dyskinesias in Parkinson’s disease: a double blind cross over study. J Neurol Neurosurg Psychiatry 75:295–297
Kim SE, Choi JY, Choe YS, Choi Y, Lee WY (2003) Serotonin transporters in the midbrain of Parkinson’s disease patients: a study with 123I-beta-CIT SPECT. J Nucl Med 44:870–876
Kish SJ, Tong J, Hornykiewicz O, Rajput A, Chang LJ, Guttman M, Furukawa Y (2008) Preferential loss of serotonin markers in caudate versus putamen in Parkinson’s disease. Brain 131:120–131
Klawans HL, Ringel SP (1973) A clinical study of methysergide in Parkinsonism: evidence against a serotonergic mechanism. J Neurol Sci 19:399–405
Kleedorfer B, Lees AJ, Stern GM (1991) Buspirone in the treatment of levodopa induced dyskinesias. J Neurol Neurosurg Psychiatry 54:376–377
Kohl Z, Winner B, Ubhi K et al (2012) Fluoxetine rescues impaired hippocampal neurogenesis in a transgenic A53T synuclein mouse model. Eur J Neurosci 35:10–19
Kuan WL, Zhao JW, Barker RA (2008) The role of anxiety in the development of levodopa-induced dyskinesias in an animal model of Parkinson’s disease, and the effect of chronic treatment with the selective serotonin reuptake inhibitor citalopram. Psychopharmacology 197:279–293
Kuno A, Ogawa T, Ikeguchi K, Fujimoto K, Nakano I (2001) Deterioration of parkinsonian symptoms and psychosis after fluvoxamine medication. Neurol Med 54:157–160
Lavoie B, Parent A (1990) Immunohistochemical study of the serotoninergic innervation of the basal ganglia in the squirrel monkey. J Comp Neurol 299:1–16
Leentjens AF, Vreeling FW, Luijckx GJ, Verhey FR (2003) SSRIs in the treatment of depression in Parkinson’s disease. Int J Geriatr Psychiatry 18:552–554
Leo RJ (1996) Movement disorders associated with the serotonin selective reuptake inhibitors. J Clin Psychiatry 57:449–454
Linazasoro G (2000) Worsening of Parkinson’s disease by citalopram. Parkinsonism Relat Disord 6:111–113
Lindgren HS, Andersson DR, Lagerkvist S, Nissbrandt H, Cenci MA (2010) L-DOPA-induced dopamine efflux in the striatum and the substantia nigra in a rat model of Parkinson’s disease: temporal and quantitative relationship to the expression of dyskinesia. J Neurochem 112:1465–1476
Lopez-Meza E, Ruiz-Chow A, Ramirez-Bermudez J (2005) Aripiprazole in psychosis associated with Parkinson’s disease. J Neuropsychiatry Clin Neurosci 17:421–422
Ludwig CL, Weinberger DR, Bruno G, Gillespie M, Bakker K, LeWitt PA, Chase TN (1986) Buspirone, Parkinson’s disease, and the locus ceruleus. Clin Neuropharmacol 9:373–378
Lundblad M, Andersson M, Winkler C, Kirik D, Wierup N, Cenci MA (2002) Pharmacological validation of behavioural measures of akinesia and dyskinesia in a rat model of Parkinson’s disease. Eur J Neurosci 15:120–132
Lundblad M, Usiello A, Carta M, Hakansson K, Fisone G, Cenci MA (2005) Pharmacological validation of a mouse model of l-DOPA-induced dyskinesia. Exp Neurol 194:66–75
Maeda T, Yoshida J, Kudo K, Nagata K (2009) Systemic L-DOPA deplete the striatal serotonin release in rats with nigro-striatal dopaminergic denervation [Abstract]. Mov Disord 24(Suppl 1):S355–S356
Maeda T, Yoshida J, Kudo K, Nagata K, Yamamoto M (2010) Systemic L-DOPA deplete the striatal serotonin release in rats with nigro-striatal dopaminergic denervation [Abstract]. Mov Disord 25(Suppl 2):S416
Maertens de Noordhout A, Delwaide PJ (1986) Open pilot trial of ritanserin in parkinsonism. Clin Neuropharmacol 9:480–484
Maloteaux JM, Luabeya MK, Laterre EC, Javoy-Agid F, Agid Y, Laduron PM (1985) S2-serotonin receptors in frontal cortex of parkinsonian patients [Abstract]. J Neurol 32(Suppl):108
Maloteaux JM, Laterre EC, Laduron PM, Javoy-Agid F, Agid Y (1988) Decrease of serotonin-S2 receptors in temporal cortex of patients with Parkinson’s disease and progressive supranuclear palsy. Mov Disord 3:255–262
Mann DM, Yates PO (1983) Pathological basis for neurotransmitter changes in Parkinson’s disease. Neuropathol Appl Neurobiol 9:3–19
Marin C, Aguilar E, Rodriguez-Oroz MC, Bartoszyk GD, Obeso JA (2009) Local administration of sarizotan into the subthalamic nucleus attenuates levodopa-induced dyskinesias in 6-OHDA-lesioned rats. Psychopharmacology 204:241–250
Marsden CD (1982) The mysterious motor function of the basal ganglia: the Robert Wartenberg Lecture. Neurology 32:514–539
McCance-Katz EF, Marek KL, Price LH (1992) Serotonergic dysfunction in depression associated with Parkinson’s disease. Neurology 42:1813–1814
Meco G, Marini S, Linfante I, Modarelli F, Agnoli A (1988) Controlled single-blind crossover study of ritanserin and placebo in L-DOPA-induced dyskinesias in Parkinson’s disease. Curr Ther Res 43:262–270
Meco G, Bonifati V, Fabrizio E, Vanacore N (1994) Worsening of parkinsonism with fluvoxamine - two cases. Hum Psychopharmacol 9:439–441
Meco G, Fabrizio E, Di Rezze S, Alessandri A, Pratesi L (2003) Mirtazapine in L-DOPA-induced dyskinesias. Clin Neuropharmacol 26:179–181
Meco G, Stirpe P, Edito F, Purcaro C, Valente M, Bernardi S, Vanacore N (2009) Aripiprazole in L-DOPA-induced dyskinesias: a one-year open-label pilot study. J Neural Transm 116:881–884
Meltzer HY, Kennedy J, Dai J, Parsa M, Riley D (1995) Plasma clozapine levels and the treatment of L-DOPA-induced psychosis in Parkinson’s disease. A high potency effect of clozapine. Neuropsychopharmacology 12:39–45
Menza MM, Palermo B, Mark M (1999) Quetiapine as an alternative to clozapine in the treatment of dopamimetic psychosis in patients with Parkinson’s disease. Ann Clin Psychiatry 11:141–144
Menza M, Marin H, Kaufman K, Mark M, Lauritano M (2004) Citalopram treatment of depression in Parkinson’s disease: the impact on anxiety, disability, and cognition. J Neuropsychiatry Clin Neurosci 16:315–319
Merims D, Balas M, Peretz C, Shabtai H, Giladi N (2006) Rater-blinded, prospective comparison: quetiapine versus clozapine for Parkinson’s disease psychosis. Clin Neuropharmacol 29:331–337
Mills R, Revell S, Bahr D, Williams H, Johnson A, Friedman JH (2008) A double-blind, placebo-controlled, dose-escalation trial of pimavanserin in Parkinson’s disease and psychosis [Abstract]. Mov Disord 23(Suppl 1):S221–S222
Montastruc JL, Fabre N, Blin O, Senard JM, Rascol O, Rascol A (1995) Does fluoxetine aggravate Parkinson’s disease? A pilot prospective study. Mov Disord 10:355–357
Morgante L, Epifanio A, Spina E et al (2002) Quetiapine versus clozapine: a preliminary report of comparative effects on dopaminergic psychosis in patients with Parkinson’s disease. Neurol Sci 23(Suppl 2):S89–S90
Muller T, Olanow CW, Nutt J, Hicking C, Laska E, Russ H (2006) The PADDY-2 study: the evaluation of sarizotan for treatment-associated dyskinesia in Parkinson’s disease patients. Mov Disord 21(Suppl. 15):S591
Munoz A, Li Q, Gardoni F et al (2008) Combined 5-HT1A and 5-HT1B receptor agonists for the treatment of L-DOPA-induced dyskinesia. Brain 131:3380–3394
Murai T, Muller U, Werheid K et al (2001) In vivo evidence for differential association of striatal dopamine and midbrain serotonin systems with neuropsychiatric symptoms in Parkinson’s disease. J Neuropsychiatry Clin Neurosci 13:222–228
Nash JF, Roth BL, Brodkin JD, Nichols DE, Gudelsky GA (1994) Effect of the R(−) and S(+) isomers of MDA and MDMA on phosphatidyl inositol turnover in cultured cells expressing 5-HT2A or 5-HT2C receptors. Neurosci Lett 177:111–115
Navailles S, Bioulac B, Gross C, De Deurwaerdere P (2010) Chronic L-DOPA therapy alters central serotonergic function and L-DOPA-induced dopamine release in a region-dependent manner in a rat model of Parkinson’s disease. Neurobiol Dis 41(2):585–590
Nevalainen N, Af Bjerken S, Lundblad M, Gerhardt GA, Stromberg I (2011) Dopamine release from serotonergic nerve fibers is reduced in L-DOPA-induced dyskinesia. J Neurochem 118(1):12–23
Nichols DE, Nichols CD (2008) Serotonin receptors. Chem Rev 108:1614–1641
Nordstrom AL, Farde L, Nyberg S, Karlsson P, Halldin C, Sedvall G (1995) D1, D2, and 5-HT2 receptor occupancy in relation to clozapine serum concentration: a PET study of schizophrenic patients. Am J Psychiatry 152:1444–1449
Oh JD, Bibbiani F, Chase TN (2002) Quetiapine attenuates levodopa-induced motor complications in rodent and primate parkinsonian models. Exp Neurol 177:557–564
Ohama E, Ikuta F (1976) Parkinson’s disease: distribution of Lewy bodies and monoamine neuron system. Acta Neuropathol 34:311–319
Olanow CW, Damier P, Goetz CG et al (2004) Multicenter, open-label, trial of sarizotan in Parkinson disease patients with levodopa-induced dyskinesias (the SPLENDID Study). Clin Neuropharmacol 27:58–62
Ostock CY, Dupre KB, Eskow Jaunarajs KL et al (2011) Role of the primary motor cortex in l-DOPA-induced dyskinesia and its modulation by 5-HT1A receptor stimulation. Neuropharmacology 61:753–760
Pact V, Giduz T (1999) Mirtazapine treats resting tremor, essential tremor, and levodopa-induced dyskinesias. Neurology 53:1154
Palhagen SE, Carlsson M, Curman E, Walinder J, Granerus AK (2008) Depressive illness in Parkinson’s disease–indication of a more advanced and widespread neurodegenerative process? Acta Neurol Scand 117:295–304
Papavasilliou PS, Cotzias GC, McDowell FH, Rosal VL, Sheehan PJ (1978) Cyproheptadine in levodopa-induced dyskinesia in parkinsonism. Clin Pharmacol Ther 23:195–198
Paquette MA, Lewis JR, Meshul CK, Johnson SW, Berger SP (2009) Effects of 5-HT1A agonism and NDMA antagonism on L-DOPA-induced dyskinesia and sensorimotor behavior in the 6-OHDA rat [Abstract]. Soc Neurosci
Paquette MA, Martinez AA, Macheda T, Giuffrida A (2010) NMDA antagonism versus 5-HT1A agonism: suppressing L-DOPA-induced dyskinesia without exacerbating parkinsonism in the 6-OHDA rat [Abstract]. Soc Neurosci
Parent A, Descarries L, Beaudet A (1981) Organization of ascending serotonin systems in the adult rat brain. A radioautographic study after intraventricular administration of [3H]5-hydroxytryptamine. Neuroscience 6:115–138
Parkinson Study Group (1999) Low-dose clozapine for the treatment of drug-induced psychosis in Parkinson’s disease. The Parkinson Study Group. N Engl J Med 340:757–763
Paulus W, Jellinger K (1991) The neuropathologic basis of different clinical subgroups of Parkinson’s disease. J Neuropathol Exp Neurol 50:743–755
Paumier KL, Siderowf AD, Auinger P et al (2012) Tricyclic antidepressants delay the need for dopaminergic therapy in early Parkinson’s disease. Mov Disord 27:880–887
Pavese N, Metta V, Bose SK, Chaudhuri KR, Brooks DJ (2010) Fatigue in Parkinson’s disease is linked to striatal and limbic serotonergic dysfunction. Brain 133:3434–3443
Politis M, Wu K, Loane C, Kiferle L, Molloy S, Brooks DJ, Piccini P (2010a) Staging of serotonergic dysfunction in Parkinson’s disease: an in vivo 11C-DASB PET study. Neurobiol Dis 40:216–221
Politis M, Wu K, Loane C, Turkheimer FE, Molloy S, Brooks DJ, Piccini P (2010b) Depressive symptoms in PD correlate with higher 5-HTT binding in raphe and limbic structures. Neurology 75:1920–1927
Pollak P, Tison F, Rascol O et al (2004) Clozapine in drug induced psychosis in Parkinson’s disease: a randomised, placebo controlled study with open follow up. J Neurol Neurosurg Psychiatry 75:689–695
Prueter C, Habermeyer B, Norra C, Kosinski CM (2003) Akathisia as a side effect of antipsychotic treatment with quetiapine in a patient with Parkinson’s disease. Mov Disord 18:712–713
Raisman R, Cash R, Agid Y (1986) Parkinson’s disease: decreased density of 3H-imipramine and 3H-paroxetine binding sites in putamen. Neurology 36:556–560
Rampello L, Chiechio S, Raffaele R, Vecchio I, Nicoletti F (2002) The SSRI, citalopram, improves bradykinesia in patients with Parkinson’s disease treated with L-DOPA. Clin Neuropharmacol 25:21–24
Rascol O, Damier P, Goetz CG et al (2006) A large phase III study to evaluate the safety and efficacy of sarizotan in the treatment of L-DOPA-induced dyskinesia associated with Parkinson’s disease: the Paddy-1 study. Mov Disord 21(Suppl. 15):S492–S493
Reddy S, Factor SA, Molho ES, Feustel PJ (2002) The effect of quetiapine on psychosis and motor function in parkinsonian patients with and without dementia. Mov Disord 17:676–681
Remy P, Doder M, Lees A, Turjanski N, Brooks D (2005) Depression in Parkinson’s disease: loss of dopamine and noradrenaline innervation in the limbic system. Brain 128:1314–1322
Riahi G, Morissette M, Parent M, Di Paolo T (2011) Brain 5-HT(2A) receptors in MPTP monkeys and levodopa-induced dyskinesias. Eur J Neurosci 33(10):1823–1831
Rich SS, Friedman JH, Ott BR (1995) Risperidone versus clozapine in the treatment of psychosis in six patients with Parkinson’s disease and other akinetic-rigid syndromes. J Clin Psychiatry 56:556–559
Richard IH, Kurlan R (1997) A survey of antidepressant drug use in Parkinson’s disease. Parkinson Study Group. Neurology 49:1168–1170
Richard IH, Kurlan R, Tanner C, Factor S, Hubble J, Suchowersky O, Waters C (1997) Serotonin syndrome and the combined use of deprenyl and an antidepressant in Parkinson’s disease. Parkinson Study Group. Neurology 48:1070–1077
Richard IH, McDermott MP, Kurlan R et al (2012) A randomized, double-blind, placebo-controlled trial of antidepressants in Parkinson disease. Neurology 78:1229–1236
Richelson E, Souder T (2000) Binding of antipsychotic drugs to human brain receptors focus on newer generation compounds. Life Sci 68:29–39
Ritter JL, Alexander B (1997) Retrospective study of selegiline-antidepressant drug interactions and a review of the literature. Ann Clin Psychiatry 9:7–13
Roberts C (2006) ACP-103, a 5-HT2A receptor inverse agonist. Curr Opin Investig Drugs 7:653–660
Rylander D, Parent M, O’Sullivan SS et al (2010) Maladaptive plasticity of serotonin axon terminals in levodopa-induced dyskinesia. Ann Neurol 68:619–628
Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y (1983) Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson’s disease. Brain Res 275:321–328
Schonfeldt-Lecuona C, Connemann BJ (2004) Aripiprazole and Parkinson’s disease psychosis. Am J Psychiatry 161:373–374
Seeman P, Tallerico T (1999) Rapid release of antipsychotic drugs from dopamine D2 receptors: an explanation for low receptor occupancy and early clinical relapse upon withdrawal of clozapine or quetiapine. Am J Psychiatry 156:876–884
Seppi K, Weintraub D, Coelho M et al (2011) The Movement Disorder Society Evidence-Based Medicine Review Update: treatments for the non-motor symptoms of Parkinson’s disease. Mov Disord 26(Suppl 3):S42–S80
Sharp SI, Ballard CG, Ziabreva I et al (2008) Cortical serotonin 1A receptor levels are associated with depression in patients with dementia with Lewy bodies and Parkinson’s disease dementia. Dement Geriatr Cogn Disord 26:330–338
Strecker K, Wegner F, Hesse S et al (2011) Preserved serotonin transporter binding in de novo Parkinson’s disease: negative correlation with the dopamine transporter. J Neurol 258:19–26
Suzuki K, Okada K, Wakuda T et al (2010) Destruction of dopaminergic neurons in the midbrain by 6-hydroxydopamine decreases hippocampal cell proliferation in rats: reversal by fluoxetine. PLoS ONE 5:e9260
Tani Y, Ogata A, Koyama M, Inoue T (2010) Effects of piclozotan (SUN N4057), a partial serotonin 1A receptor agonist, on motor complications induced by repeated administration of levodopa in parkinsonian rats. Eur J Pharmacol 649:218–223
Tesei S, Antonini A, Canesi M, Zecchinelli A, Mariani CB, Pezzoli G (2000) Tolerability of paroxetine in Parkinson’s disease: a prospective study. Mov Disord 15:986–989
Thomas AA, Friedman JH (2010) Current use of clozapine in Parkinson disease and related disorders. Clin Neuropharmacol 33:14–16
Trappler B, Friedman S (1998) Treatment of depression in Parkinson’s disease in the very old. J Pharm Tech 14:110–115
Trosch RM, Group CC (1996) Clozapine use in Parkinson’s disease [Abstract]. Neurology 46:A375–A376
Trosch RM, Friedman JH, Lannon MC et al (1998) Clozapine use in Parkinson’s disease: a retrospective analysis of a large multicentered clinical experience. Mov Disord 13:377–382
Vanover KE, Weiner DM, Makhay M et al (2006) Pharmacological and behavioral profile of N-(4-fluorophenylmethyl)-N-(1-methylpiperidin-4-yl)-N′-(4-(2-methylpropylo xy)phenylmethyl) carbamide (2R,3R)-dihydroxybutanedioate (2:1) (ACP-103), a novel 5-hydroxytryptamine(2A) receptor inverse agonist. J Pharmacol Exp Ther 317:910–918
Vanover KE, Betz AJ, Weber SM et al (2008) A 5-HT2A receptor inverse agonist, ACP-103, reduces tremor in a rat model and levodopa-induced dyskinesias in a monkey model. Pharmacol Biochem Behav 90:540–544
Visanji NP, Gomez-Ramirez J, Johnston TH, Pires D, Voon V, Brotchie JM, Fox SH (2006) Pharmacological characterization of psychosis-like behavior in the MPTP-lesioned nonhuman primate model of Parkinson’s disease. Mov Disord 21:1879–1891
Weiner WJ, Minagar A, Shulman LM (2000) Quetiapine for l-DOPA-induced psychosis in PD. Neurology 54:1538
Wermuth L, Sorensen PS, Timm S et al (1998) Depression in idiopathic Parkinson’s disease treated with citalopram—A placebo-controlled trial. Nord J Psychiatry 52:163–169
Wickremaratchi M, Morris HR, Ali IM (2006) Aripiprazole associated with severe exacerbation of Parkinson’s disease. Mov Disord 21:1538–1539
Wilson JM, Levey AI, Rajput A et al (1996) Differential changes in neurochemical markers of striatal dopamine nerve terminals in idiopathic Parkinson’s disease. Neurology 47:718–726
Acknowledgments
The present work was supported by The Cure Parkinson Trust and Krembil Neuroscience Fund.
Conflict of interest
There are no financial disclosures relevant to this review article and no conflict of interest. SHF has received consultancy fees from Merck, Merck Serono, Teva. PH has received consultancy fees from Atuka Inc.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huot, P., Fox, S.H. The serotonergic system in motor and non-motor manifestations of Parkinson’s disease. Exp Brain Res 230, 463–476 (2013). https://doi.org/10.1007/s00221-013-3621-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-013-3621-2